Efficacy and Safety of Dual Targeted Therapy for Partially or Non-responsive Inflammatory Bowel Disease: A Systematic Review of the Literature
- PMID: 36807832
- PMCID: PMC9942632
- DOI: 10.1007/s10620-023-07837-0
Efficacy and Safety of Dual Targeted Therapy for Partially or Non-responsive Inflammatory Bowel Disease: A Systematic Review of the Literature
Erratum in
-
Correction to: Efficacy and Safety of Dual Targeted Therapy for Partially or Non‑responsive Inflammatory Bowel Disease: A Systematic Review of the Literature.Dig Dis Sci. 2023 Dec;68(12):4540. doi: 10.1007/s10620-023-08130-w. Dig Dis Sci. 2023. PMID: 37891442 Free PMC article. No abstract available.
Abstract
Background: Dual targeted therapy (DTT) has emerged as an attractive therapeutic option for select patients with active inflammatory bowel disease (IBD) who are unable to achieve remission with biologic or small molecule monotherapy. We conducted a systematic review of specific DTT combinations in patients with IBD.
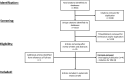
Methods: We conducted a systematic search of MEDLINE, EMBASE, Scopus, CINAHL Complete, Web of Science Core Collection, and Cochrane Library to identify articles related to the use of DTT for the treatment of Crohn Disease (CD) or ulcerative colitis (UC) published before February 2021.
Results: Twenty-nine studies were identified comprising 288 patients started on DTT for partially or non-responsive IBD. We identified 14 studies with 113 patients receiving anti-tumor necrosis factor (TNF) and anti-integrin therapies (i.e., vedolizumab and natalizumab), 12 studies with 55 patients receiving vedolizumab and ustekinumab, nine studies with 68 patients receiving vedolizumab and tofacitinib, five studies with 24 patients receiving anti-TNF therapy and tofacitinib, six studies with 18 patients receiving anti-TNF therapy and ustekinumab, and three studies with 13 patients receiving ustekinumab and tofacitinib.
Conclusion: DTT is a promising approach to improve IBD treatment for patients with incomplete responses to targeted monotherapy. Larger prospective clinical studies are needed to confirm these findings as is additional predictive modeling to identify the patient subgroups most likely to require and benefit from this approach.
Keywords: Biologics; Combination therapy; Inflammatory bowel disease; Therapeutics.
© 2023. The Author(s), under exclusive licence to Springer Science+Business Media, LLC, part of Springer Nature.
Conflict of interest statement
PDRH received consulting fees from AbbVie, Amgen, Genentech, and Pfizer. RWS received consulting fees from AbbVie, Janssen, Takeda, Merck, Gilead, Eli Lily, Exact Science, Evergreen Pharmaceuticals, Corrona LLC. JAB received consulting fees from BUHLMANN Diagnostics Corp. All other authors report no relevant disclosures.
References
-
- Reinink AR, Lee TC, Higgins PD. Endoscopic Mucosal Healing Predicts Favorable Clinical Outcomes in Inflammatory Bowel Disease: A Meta-analysis. Inflamm Bowel Dis. 22(8):1859–69. 10.1097/MIB.0000000000000816 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical