ABCA3-related interstitial lung disease beyond infancy
- PMID: 36808083
- PMCID: PMC10314027
- DOI: 10.1136/thorax-2022-219434
ABCA3-related interstitial lung disease beyond infancy
Abstract
Background: The majority of patients with childhood interstitial lung disease (chILD) caused by pathogenic variants in ATP binding cassette subfamily A member 3 (ABCA3) develop severe respiratory insufficiency within their first year of life and succumb to disease if not lung transplanted. This register-based cohort study reviews patients with ABCA3 lung disease who survived beyond the age of 1 year.
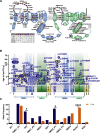
Method: Over a 21-year period, patients diagnosed as chILD due to ABCA3 deficiency were identified from the Kids Lung Register database. 44 patients survived beyond the first year of life and their long-term clinical course, oxygen supplementation and pulmonary function were reviewed. Chest CT and histopathology were scored blindly.
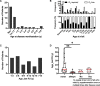
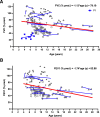
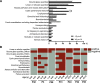
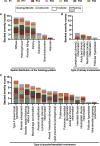
Results: At the end of the observation period, median age was 6.3 years (IQR: 2.8-11.7) and 36/44 (82%) were still alive without transplantation. Patients who had never received supplemental oxygen therapy survived longer than those persistently required oxygen supplementation (9.7 (95% CI 6.7 to 27.7) vs 3.0 years (95% CI 1.5 to 5.0), p=0.0126). Interstitial lung disease was clearly progressive over time based on lung function (forced vital capacity % predicted absolute loss -1.1% /year) and on chest CT (increasing cystic lesions in those with repetitive imaging). Lung histology pattern were variable (chronic pneumonitis of infancy, non-specific interstitial pneumonia, and desquamative interstitial pneumonia). In 37/44 subjects, the ABCA3 sequence variants were missense variants, small insertions or deletions with in-silico tools predicting some residual ABCA3 transporter function.
Conclusion: The natural history of ABCA3-related interstitial lung disease progresses during childhood and adolescence. Disease-modifying treatments are desirable to delay such disease course.
Keywords: ABCA3; paediatric interstitial lung disease; rare lung diseases.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
