Characterization of Fragile X Mental Retardation Protein expression in human nociceptors and their axonal projections to the spinal dorsal horn
- PMID: 36808110
- PMCID: PMC10038933
- DOI: 10.1002/cne.25463
Characterization of Fragile X Mental Retardation Protein expression in human nociceptors and their axonal projections to the spinal dorsal horn
Abstract
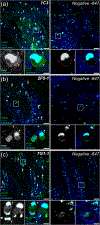
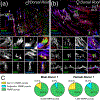
Fragile X Mental Retardation Protein (FMRP) regulates activity-dependent RNA localization and local translation to modulate synaptic plasticity throughout the central nervous system. Mutations in the FMR1 gene that hinder or ablate FMRP function cause Fragile X Syndrome (FXS), a disorder associated with sensory processing dysfunction. FXS premutations are associated with increased FMRP expression and neurological impairments including sex dimorphic presentations of chronic pain. In mice, FMRP ablation causes dysregulated dorsal root ganglion (DRG) neuron excitability and synaptic vesicle exocytosis, spinal circuit activity, and decreased translation-dependent nociceptive sensitization. Activity-dependent, local translation is a key mechanism for enhancing primary nociceptor excitability that promotes pain in animals and humans. These works indicate that FMRP likely regulates nociception and pain at the level of the primary nociceptor or spinal cord. Therefore, we sought to better understand FMRP expression in the human DRG and spinal cord using immunostaining in organ donor tissues. We find that FMRP is highly expressed in DRG and spinal neuron subsets with substantia gelatinosa exhibiting the most abundant immunoreactivity in spinal synaptic fields. Here, it is expressed in nociceptor axons. FMRP puncta colocalized with Nav1.7 and TRPV1 receptor signals suggesting a pool of axoplasmic FMRP localizes to plasma membrane-associated loci in these branches. Interestingly, FMRP puncta exhibited notable colocalization with calcitonin gene-related peptide (CGRP) immunoreactivity selectively in female spinal cord. Our results support a regulatory role for FMRP in human nociceptor axons of the dorsal horn and implicate it in the sex dimorphic actions of CGRP signaling in nociceptive sensitization and chronic pain.
© 2023 Wiley Periodicals LLC.
Conflict of interest statement
Conflict of Interest Statement
The authors declare that they have no conflicts of interest related to this work.
Figures




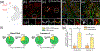
Similar articles
-
Convergence of peptidergic and non-peptidergic protein markers in the human dorsal root ganglion and spinal dorsal horn.J Comp Neurol. 2021 Jul 1;529(10):2771-2788. doi: 10.1002/cne.25122. Epub 2021 Feb 16. J Comp Neurol. 2021. PMID: 33550628 Free PMC article.
-
Selective Deletion of Astroglial FMRP Dysregulates Glutamate Transporter GLT1 and Contributes to Fragile X Syndrome Phenotypes In Vivo.J Neurosci. 2016 Jul 6;36(27):7079-94. doi: 10.1523/JNEUROSCI.1069-16.2016. J Neurosci. 2016. PMID: 27383586 Free PMC article.
-
Neuron-Specific FMRP Roles in Experience-Dependent Remodeling of Olfactory Brain Innervation during an Early-Life Critical Period.J Neurosci. 2021 Feb 10;41(6):1218-1241. doi: 10.1523/JNEUROSCI.2167-20.2020. Epub 2021 Jan 5. J Neurosci. 2021. PMID: 33402421 Free PMC article.
-
The molecular biology of FMRP: new insights into fragile X syndrome.Nat Rev Neurosci. 2021 Apr;22(4):209-222. doi: 10.1038/s41583-021-00432-0. Epub 2021 Feb 19. Nat Rev Neurosci. 2021. PMID: 33608673 Free PMC article. Review.
-
FMRP ribonucleoprotein complexes and RNA homeostasis.Adv Genet. 2020;105:95-136. doi: 10.1016/bs.adgen.2020.01.001. Epub 2020 Feb 6. Adv Genet. 2020. PMID: 32560791 Review.
Cited by
-
Case Series: Vestibular Migraines in Fragile X Premutation Carriers.J Clin Med. 2024 Jan 16;13(2):504. doi: 10.3390/jcm13020504. J Clin Med. 2024. PMID: 38256638 Free PMC article.
-
Deciphering the molecular landscape of human peripheral nerves: implications for diabetic peripheral neuropathy.bioRxiv [Preprint]. 2024 Jun 16:2024.06.15.599167. doi: 10.1101/2024.06.15.599167. bioRxiv. 2024. PMID: 38915676 Free PMC article. Preprint.
-
Presynaptic Protein Synthesis in Brain Function and Disease.J Neurosci. 2023 Nov 8;43(45):7483-7488. doi: 10.1523/JNEUROSCI.1454-23.2023. J Neurosci. 2023. PMID: 37940588 Free PMC article.
-
Interleukin-6 induces nascent protein synthesis in human dorsal root ganglion nociceptors primarily via MNK-eIF4E signaling.Neurobiol Pain. 2024 Jul 26;16:100159. doi: 10.1016/j.ynpai.2024.100159. eCollection 2024 Jul-Dec. Neurobiol Pain. 2024. PMID: 39156884 Free PMC article.
-
Decreased KCC2 expression in the human spinal dorsal horn associated with chronic pain and long-term opioid use.Pain. 2025 Jul 11:10.1097/j.pain.0000000000003700. doi: 10.1097/j.pain.0000000000003700. Online ahead of print. Pain. 2025. PMID: 40667986
References
-
- Akins MR, Berk-Rauch HE, Kwan KY, Mitchell ME, Shepard KA, Korsak LIT, Stackpole EE, Warner-Schmidt JL, Sestan N, Cameron HA, & Fallon JR (2017). Axonal ribosomes and mRNAs associate with fragile X granules in adult rodent and human brains. Human Molecular Genetics, 26(1), 192–209. 10.1093/hmg/ddw381 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

