Nivolumab with or without ipilimumab in pediatric patients with high-grade CNS malignancies: Safety, efficacy, biomarker, and pharmacokinetics-CheckMate 908
- PMID: 36808285
- PMCID: PMC10398811
- DOI: 10.1093/neuonc/noad031
Nivolumab with or without ipilimumab in pediatric patients with high-grade CNS malignancies: Safety, efficacy, biomarker, and pharmacokinetics-CheckMate 908
Abstract
Background: Therapeutic options are limited in pediatric CNS malignancies. CheckMate 908 (NCT03130959) is an open-label, sequential-arm, phase 1b/2 study investigating nivolumab (NIVO) and NIVO + ipilimumab (IPI) in pediatric patients with high-grade CNS malignancies.
Methods: Patients (N = 166) in 5 cohorts received NIVO 3 mg/kg every 2 weeks (Q2W) or NIVO 3 mg/kg + IPI 1 mg/kg every 3 weeks (4 doses) followed by NIVO 3 mg/kg Q2W. Primary endpoints included overall survival (OS; newly diagnosed diffuse intrinsic pontine glioma [DIPG]) and progression-free survival (PFS; other recurrent/progressive or relapsed/resistant CNS cohorts). Secondary endpoints included other efficacy metrics and safety. Exploratory endpoints included pharmacokinetics and biomarker analyses.
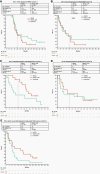
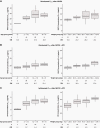
Results: As of January 13, 2021, median OS (80% CI) was 11.7 (10.3-16.5) and 10.8 (9.1-15.8) months with NIVO and NIVO + IPI, respectively, in newly diagnosed DIPG. Median PFS (80% CI) with NIVO and NIVO + IPI was 1.7 (1.4-2.7) and 1.3 (1.2-1.5) months, respectively, in recurrent/progressive high-grade glioma; 1.4 (1.2-1.4) and 2.8 (1.5-4.5) months in relapsed/resistant medulloblastoma; and 1.4 (1.4-2.6) and 4.6 (1.4-5.4) months in relapsed/resistant ependymoma. In patients with other recurrent/progressive CNS tumors, median PFS (95% CI) was 1.2 (1.1-1.3) and 1.6 (1.3-3.5) months, respectively. Grade 3/4 treatment-related adverse-event rates were 14.1% (NIVO) and 27.2% (NIVO + IPI). NIVO and IPI first-dose trough concentrations were lower in youngest and lowest-weight patients. Baseline tumor programmed death ligand 1 expression was not associated with survival.
Conclusions: NIVO ± IPI did not demonstrate clinical benefit relative to historical data. The overall safety profiles were manageable with no new safety signals.
Keywords: DIPG; HGG; checkpoint inhibitors; ependymoma; medulloblastoma.
© The Author(s) 2023. Published by Oxford University Press on behalf of the Society for Neuro-Oncology.
Conflict of interest statement
I.J.D. received grant funding from Genentech/Roche and Novartis; personal fees from AstraZeneca, Bristol Myers Squibb, Celgene, a Bristol-Myers Squibb company, Day One, Fennec, Pyramid, QED, Roche. F.D. received fees for advisory board roles from Bayer, Bristol Myers Squibb, Roche, Celgene, a Bristol-Myers Squibb company, LOXO Oncology, Servier, Tesaro; travel expenses from Bayer, Bristol Myers Squibb, Roche; and consultancy roles from Servier. (Note: all honoraria were contributed to an account at Institut Curie, not his personal funds). D.H. received funding from the National Institute for Health and Care Research Great Ormond Street Hospital Biomedical Research Centre; consultancy fees from AstraZeneca, Bayer, Roche, LOXO Oncology, Novartis. His views are his own and not necessarily those of the NHS, the NIHR or the Department of Health, London, UK. A.L. received fees from Servier and Gilead; served on advisory boards for Jazz Pharmaceuticals and Servier. N.A. received grant funding from Bristol Myers Squibb; fees from Bristol Myers Squibb, Ankira, Bayer, Roche. M.E. received fees from and served on an advisory board for Bristol Myers Squibb. A.G. served on an advisory board for Day One Therapeutics. D.W., R.T., and L.Z. are employees of and received stock from Bristol Myers Squibb. Y.W. is an employee of Bristol Myers Squibb. K.C. received grant funding from Regeneron and Novartis; fees from DNAtrix, CRICO, Roetzel & Andres; DMSC member for Y-mAbs. J.R.H. received honoraria for consultation from Bayer Australia, Alexion Pharmaceuticals, Boxer Capital; N.K.F., L.H., T.H., S.G., U.B., and M.P. have nothing to disclose.
Figures


References
-
- Miller KD, Ostrom QT, Kruchko C, et al. . Brain and other central nervous system tumor statistics, 2021. CA Cancer J Clin. 2021; 71(5):381–406. - PubMed
-
- Johnston DL, Keene D, Strother D, et al. . Survival following tumor recurrence in children with medulloblastoma. J Pediatr Hematol Oncol. 2018; 40(3):e159–e163. - PubMed
-
- Ritzmann TA, Rogers HA, Paine SML, et al. . A retrospective analysis of recurrent pediatric ependymoma reveals extremely poor survival and ineffectiveness of current treatments across central nervous system locations and molecular subgroups. Pediatric Blood Cancer. 2020; 67(9):e28426. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

