The transcriptional landscape of plant infection by the rice blast fungus Magnaporthe oryzae reveals distinct families of temporally co-regulated and structurally conserved effectors
- PMID: 36808541
- PMCID: PMC10118281
- DOI: 10.1093/plcell/koad036
The transcriptional landscape of plant infection by the rice blast fungus Magnaporthe oryzae reveals distinct families of temporally co-regulated and structurally conserved effectors
Abstract
The rice blast fungus Magnaporthe oryzae causes a devastating disease that threatens global rice (Oryza sativa) production. Despite intense study, the biology of plant tissue invasion during blast disease remains poorly understood. Here we report a high-resolution transcriptional profiling study of the entire plant-associated development of the blast fungus. Our analysis revealed major temporal changes in fungal gene expression during plant infection. Pathogen gene expression could be classified into 10 modules of temporally co-expressed genes, providing evidence for the induction of pronounced shifts in primary and secondary metabolism, cell signaling, and transcriptional regulation. A set of 863 genes encoding secreted proteins are differentially expressed at specific stages of infection, and 546 genes named MEP (Magnaportheeffector protein) genes were predicted to encode effectors. Computational prediction of structurally related MEPs, including the MAX effector family, revealed their temporal co-regulation in the same co-expression modules. We characterized 32 MEP genes and demonstrate that Mep effectors are predominantly targeted to the cytoplasm of rice cells via the biotrophic interfacial complex and use a common unconventional secretory pathway. Taken together, our study reveals major changes in gene expression associated with blast disease and identifies a diverse repertoire of effectors critical for successful infection.
© The Author(s) 2023. Published by Oxford University Press on behalf of American Society of Plant Biologists.
Conflict of interest statement
Conflict of interest statement. The authors declare no competing interests.
Figures


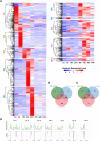

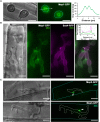
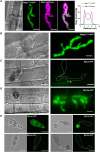
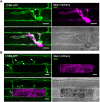
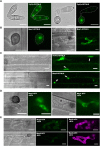
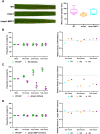
Comment in
-
Reeling in countless effectors (RICE): time-course transcriptomics of rice blast disease reveal an expanded effector repertoire for Magnaporthe oryzae.Plant Cell. 2023 Apr 20;35(5):1290-1291. doi: 10.1093/plcell/koad035. Plant Cell. 2023. PMID: 36780918 Free PMC article. No abstract available.
References
-
- Cao H, Huang P, Zhang L, Shi Y, Sun D, Yan Y, Liu X, Dong B, Chen G, Snyder JH, et al. . Characterization of 47 Cys2 -His2 zinc finger proteins required for the development and pathogenicity of the rice blast fungus Magnaporthe oryzae. New Phytol. 2016:211(3):1035–1051. 10.1111/nph.13948 - DOI - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

