The neuroendocrine stress response compensates for suppression of insulin secretion by volatile anesthetic agents: An observational study
- PMID: 36808704
- PMCID: PMC9937792
- DOI: 10.14814/phy2.15603
The neuroendocrine stress response compensates for suppression of insulin secretion by volatile anesthetic agents: An observational study
Abstract
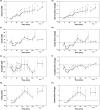
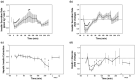
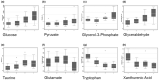
Alterations in perioperative metabolic function, particularly hyperglycemia, are associated with increased post-operative complications, even in patients without preexisting metabolic abnormalities. Anesthetic medications and the neuroendocrine stress response to surgery may both contribute to altered energy metabolism through impaired glucose and insulin homeostasis but the discrete pathways involved are unclear. Prior human studies, though informative, have been limited by analytic sensitivity or technique, preventing resolution of underlying mechanisms. We hypothesized that general anesthesia with a volatile agent would suppress basal insulin secretion without altering hepatic insulin extraction, and that surgical stress would promote hyperglycemia through gluconeogenesis, lipid oxidation, and insulin resistance. In order to address these hypotheses, we conducted an observational study of subjects undergoing multi-level lumbar surgery with an inhaled anesthetic agent. We measured circulating glucose, insulin, c-peptide, and cortisol frequently throughout the perioperative period and analyzed the circulating metabolome in a subset of these samples. We found volatile anesthetic agents suppress basal insulin secretion and uncouple glucose-stimulated insulin secretion. Following surgical stimulus, this inhibition disappeared and there was gluconeogenesis with selective amino acid metabolism. No robust evidence of lipid metabolism or insulin resistance was observed. These results show that volatile anesthetic agents suppress basal insulin secretion, which results in reduced glucose metabolism. The neuroendocrine stress response to surgery ameliorates the inhibitory effect of the volatile agent on insulin secretion and glucose metabolism, promoting catabolic gluconeogenesis. A better understanding of the complex metabolic interaction between anesthetic medications and surgical stress is needed to inform design of clinical pathways aimed at improving perioperative metabolic function.
© 2023 The Authors. Physiological Reports published by Wiley Periodicals LLC on behalf of The Physiological Society and the American Physiological Society.
Conflict of interest statement
No authors have conflicts of interest to declare related to this study.
Figures



References
-
- Abdelmalak, B. B. , Knittel, J. , Abdelmalak, J. B. , Dalton, J. E. , Christiansen, E. , Foss, J. , Argalious, M. , Zimmerman, R. , & van den Berghe, G. (2014). Preoperative blood glucose concentrations and postoperative outcomes after elective non‐cardiac surgery: An observational study. British Journal of Anaesthesia, 112, 79–88. - PubMed
-
- Akhtar, S. , Barash, P. G. , & Inzucchi, S. E. (2010). Scientific principles and clinical implications of perioperative glucose regulation and control. Anesthesia and Analgesia, 110, 478–497. - PubMed
-
- Bagry, H. S. , Raghavendran, S. , & Carli, F. (2008). Metabolic syndrome and insulin resistance: Perioperative considerations. Anesthesiology, 108, 506–523. - PubMed
-
- Baker, M. T. , & Naguib, M. (2005). Propofol: The challenges of formulation. Anesthesiology, 103, 860–876. - PubMed
-
- Carli, F. , Galeone, M. , Gzodzic, B. , Hong, X. , Fried, G. M. , Wykes, L. , Eberhart, L. , & Schricker, T. (2005). Effect of laparoscopic colon resection on postoperative glucose utilization and protein sparing: An integrated analysis of glucose and protein metabolism during the fasted and fed states using stable isotopes. Archives of Surgery, 140, 593–597. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

