Genes4Epilepsy: An epilepsy gene resource
- PMID: 36808730
- PMCID: PMC10952165
- DOI: 10.1111/epi.17547
Genes4Epilepsy: An epilepsy gene resource
Abstract
Objective: "How many epilepsy genes are there?" is a frequently asked question. We sought to (1) provide a curated list of genes that cause monogenic epilepsies, and (2) compare and contrast epilepsy gene panels from multiple sources.
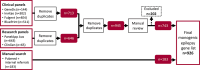
Methods: We compared genes included on the epilepsy panels (as of July 29, 2022) of four clinical diagnostic providers: Invitae, GeneDx, Fulgent Genetics, and Blueprint Genetics; and two research resources: PanelApp Australia and ClinGen. A master list of all unique genes was supplemented by additional genes identified via PubMed searches up until August 15, 2022, using the search terms "genetics" AND/OR "epilepsy" AND/OR "seizures". Evidence supporting a monogenic role for all genes was manually reviewed; those with limited or disputed evidence were excluded. All genes were annotated according to inheritance pattern and broad epilepsy phenotype.
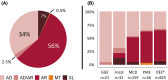
Results: The comparison of genes included on epilepsy clinical panels revealed high heterogeneity in both number of genes (range: 144-511) and content. Just 111 genes (15.5%) were included on all four clinical panels. Subsequent manual curation of all "epilepsy genes" identified >900 monogenic etiologies. Almost 90% of genes were associated with developmental and epileptic encephalopathies. By comparison only 5% of genes were associated with monogenic causes of "common epilepsies" (i.e., generalized and focal epilepsy syndromes). Autosomal recessive genes were most frequent (56% of genes); however, this varied according to the associated epilepsy phenotype(s). Genes associated with common epilepsy syndromes were more likely to be dominantly inherited and associated with multiple epilepsy types.
Significance: Our curated list of monogenic epilepsy genes is publicly available: github.com/bahlolab/genes4epilepsy and will be regularly updated. This gene resource can be utilized to target genes beyond those included on clinical gene panels, for gene enrichment methods and candidate gene prioritization. We invite ongoing feedback and contributions from the scientific community via genes4-epilepsy@unimelb.edu.au.
Keywords: epilepsy panel; genetic architecture; monogenic disease; pathogenic gene resource.
© 2023 The Authors. Epilepsia published by Wiley Periodicals LLC on behalf of International League Against Epilepsy.
Conflict of interest statement
SFB has received unrestricted educational grants from UCB Pharma, Chiesi, Liva Nova, and Seer Medical; and personal fees from Sequirus and Praxis during the conduct of this study. Ingrid Scheffer has served on scientific advisory boards for BioMarin, Chiesi, Eisai, Encoded Therapeutics, GlaxoSmithKline, Knopp Biosciences, Nutricia, Rogcon, Takeda Pharmaceuticals, UCB, Xenon and Pharmaceuticals; has received speaker honoraria from GlaxoSmithKline, UCB, BioMarin, Biocodex, Chiesi, Liva Nova, Nutricia, Zuellig Pharma, and Eisai; has received funding for travel from UCB, Biocodex, GlaxoSmithKline, Biomarin, and Eisai; has served as an investigator for Anavex Life Sciences, Cerecin Inc, Cerevel Therapeutics, Eisai, Encoded Therapeutics, EpiMinder Inc, Epygenyx, ES‐Therapeutics, GW Pharma, Marinus, Neurocrine BioSciences, Ovid Therapeutics, Takeda Pharmaceuticals, UCB, Ultragenyx, Xenon Pharmaceuticals, Zogenix, and Zynerba; has consulted for Care Beyond Diagnosis, Epilepsy Consortium, Atheneum Partners, Ovid Therapeutics, UCB, Zynerba Pharmaceuticals, BioMarin, Encoded Therapeutics, and Biohaven Pharmaceuticals; and is a Non‐Executive Director of Bellberry Ltd and a Director of the Australian Academy of Health and Medical Sciences and the Australian Council of Learned Academies Limited. None of the remaining authors has any conflict of interest to disclose.
Figures

References
-
- Steinlein OK, Mulley JC, Propping P, Wallace RH, Phillips HA, Sutherland GR, et al. A missense mutation in the neuronal nicotinic acetylcholine receptor alpha 4 subunit is associated with autosomal dominant nocturnal frontal lobe epilepsy. Nat Genet. 1995;11:201–3. - PubMed
-
- International Human Genome Sequencing Consortium . Finishing the euchromatic sequence of the human genome. Nature. 2004;21(431):931–45. - PubMed
-
- Heyne HO, Singh T, Stamberger H, Abou Jamra R, Caglayan H, Craiu D, et al. De novo variants in neurodevelopmental disorders with epilepsy. Nat Genet. 2018;50:1048–53. - PubMed
-
- Lemke JR, Riesch E, Scheurenbrand T, Schubach M, Wilhelm C, Steiner I, et al. Targeted next generation sequencing as a diagnostic tool in epileptic disorders. Epilepsia. 2012;53:1387–98. - PubMed

