Comparison between mono-tacrolimus and mono-glucocorticoid in the treatment of myasthenia gravis
- PMID: 36808840
- PMCID: PMC10109324
- DOI: 10.1002/acn3.51746
Comparison between mono-tacrolimus and mono-glucocorticoid in the treatment of myasthenia gravis
Abstract
Objective: Use of tacrolimus in mild to moderate myasthenia gravis (MG) is generally limited to glucocorticoid-refractory cases; the advantage of mono-tacrolimus over mono-glucocorticoids is unknown.
Methods: We included mild to moderate MG patients treated with mono-tacrolimus (mono-TAC) or mono-glucocorticoids (mono-GC). The correlation between the immunotherapy options and the treatment efficacy and side effects were examined in 1:1 propensity-score matching. The main outcome was time to minimal manifestations status or better (MMS or better). Secondary outcomes include time to relapse, the mean changes in Myasthenia Gravis-specific Activities of Daily Living (MG-ADL) scores and the rate of adverse events.
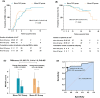
Results: Baseline characteristics showed no difference between matched groups (49 matched pairs). There were no differences in median time to MMS or better between the mono-TAC group and mono-GC group (5.1 vs. 2.8 months: unadjusted hazard ratio [HR], 0.73; 95% CI, 0.46-1.16; p = 0.180), as well as in median time to relapse (data unavailable for the mono-TAC group since 44 of 49 [89.8%] participants remained in MMS or better; 39.7 months in mono-GC group: unadjusted HR, 0.67; 95% CI, 0.23-1.97; p = 0.464). Changes in MG-ADL scores between the two groups were similar (mean differences, 0.3; 95% CI, -0.4 to 1.0; p = 0.462). The rate of adverse events was lower in the mono-TAC group compared to the mono-GC group (24.5% vs. 55.1%, p = 0.002).
Interpretation: Mono-tacrolimus performs superior tolerability with non-inferior efficacy compared to mono-glucocorticoids in mild to moderate myasthenia gravis patients who refuse or have a contraindication to glucocorticoids.
© 2023 The Authors. Annals of Clinical and Translational Neurology published by Wiley Periodicals LLC on behalf of American Neurological Association.
Conflict of interest statement
The authors declare no financial or other conflicts of interest.
Figures

References
-
- Fang W, Li Y, Mo R, et al. Hospital and healthcare insurance system record‐based epidemiological study of myasthenia gravis in southern and northern China. Neurol Sci. 2020;41(5):1211‐1223. - PubMed
-
- Gotterer L, Li Y. Maintenance immunosuppression in myasthenia gravis. J Neurol Sci. 2016;369:294‐302. - PubMed
-
- Fardet L, Flahault A, Kettaneh A, et al. Corticosteroid‐induced clinical adverse events: frequency, risk factors and patient's opinion. Br J Dermatol. 2007;157(1):142‐148. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

