Engineered Bacterial Outer Membrane Vesicles with Lipidated Heterologous Antigen as an Adjuvant-Free Vaccine Platform for Streptococcus suis
- PMID: 36809058
- PMCID: PMC10057044
- DOI: 10.1128/aem.02047-22
Engineered Bacterial Outer Membrane Vesicles with Lipidated Heterologous Antigen as an Adjuvant-Free Vaccine Platform for Streptococcus suis
Abstract
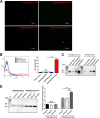
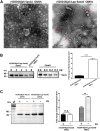
Bacterial outer membrane vesicles (OMVs) are considered a promising vaccine platform for their high built-in adjuvanticity and ability to efficiently induce immune responses. OMVs can be engineered with heterologous antigens based on genetic engineering strategies. However, several critical issues should still be validated, including optimal exposure to the OMV surface, increased production of foreign antigens, nontoxicity, and induction of powerful immune protection. In this study, engineered OMVs with the lipoprotein transport machinery (Lpp) were designed to present SaoA antigen as a vaccine platform against Streptococcus suis. The results suggest that Lpp-SaoA fusions can be delivered on the OMV surface and do not have significant toxicity. Moreover, they can be engineered as lipoprotein and significantly accumulated in OMVs at high levels, thus accounting for nearly 10% of total OMV proteins. Immunization with OMVs containing Lpp-SaoA fusion antigen induced strong specific antibody responses and high levels of cytokines, as well as a balanced Th1/Th2 immune response. Furthermore, the decorated OMV vaccination significantly enhanced microbial clearance in a mouse infection model. It was found that antiserum against lipidated OMVs significantly promoted the opsonophagocytic uptake of S. suis in RAW246.7 macrophages. Lastly, OMVs engineered with Lpp-SaoA induced 100% protection against a challenge with 8× the 50% lethal dose (LD50) of S. suis serotype 2 and 80% protection against a challenge with 16× the LD50 in mice. Altogether, the results of this study provide a promising versatile strategy for the engineering of OMVs and suggest that Lpp-based OMVs may be a universal adjuvant-free vaccine platform for important pathogens. IMPORTANCE Bacterial outer membrane vesicles (OMVs) have become a promising vaccine platform due to their excellent built-in adjuvanticity properties. However, the location and amount of the expression of the heterologous antigen in the OMVs delivered by the genetic engineering strategies should be optimized. In this study, we exploited the lipoprotein transport pathway to engineer OMVs with heterologous antigen. Not only did lapidated heterologous antigen accumulate in the engineered OMV compartment at high levels, but also it was engineered to be delivered on the OMV surface, thus leading to the optimal activation of antigen-specific B cells and T cells. Immunization with engineered OMVs induced a strong antigen-specific antibodies in mice and conferred 100% protection against S. suis challenge. In general, the data of this study provide a versatile strategy for the engineering of OMVs and suggest that OMVs engineered with lipidated heterologous antigens may be a vaccine platform for significant pathogens.
Keywords: Streptococcus suis; heterologous antigens; outer membrane vesicles (OMVs); protective immunity; vaccine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

