New Insights into the Bacterial Targets of Antimicrobial Blue Light
- PMID: 36809152
- PMCID: PMC10101057
- DOI: 10.1128/spectrum.02833-22
New Insights into the Bacterial Targets of Antimicrobial Blue Light
Abstract
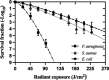
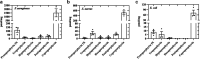
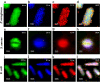
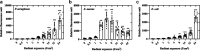
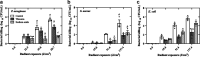
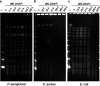
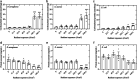
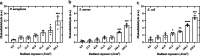
Antimicrobial blue light (aBL) offers efficacy and safety in treating infections. However, the bacterial targets for aBL are still poorly understood and may be dependent on bacterial species. Here, we investigated the biological targets of bacterial killing by aBL (λ = 410 nm) on three pathogens: Staphylococcus aureus, Escherichia coli, and Pseudomonas aeruginosa. Initially, we evaluated the killing kinetics of bacteria exposed to aBL and used this information to calculate the lethal doses (LD) responsible for killing 90 and 99.9% of bacteria. We also quantified endogenous porphyrins and assessed their spatial distribution. We then quantified and suppressed reactive oxygen species (ROS) production in bacteria to investigate their role in bacterial killing by aBL. We also assessed aBL-induced DNA damage, protein carbonylation, lipid peroxidation, and membrane permeability in bacteria. Our data showed that P. aeruginosa was more susceptible to aBL (LD99.9 = 54.7 J/cm2) relative to S. aureus (LD99.9 = 158.9 J/cm2) and E. coli (LD99.9 = 195 J/cm2). P. aeruginosa exhibited the highest concentration of endogenous porphyrins and level of ROS production relative to the other species. However, unlike other species, DNA degradation was not observed in P. aeruginosa. Sublethal doses of blue light (<LD90) could damage the cell membrane in Gram-negative species but not in S. aureus. In all bacteria, oxidative damage to bacterial DNA (except P. aeruginosa), proteins, and lipids occurred after high aBL exposures (>LD99.9). We conclude that the primary targets of aBL depend on the species, which are probably driven by variable antioxidant and DNA-repair mechanisms. IMPORTANCE Antimicrobial-drug development is facing increased scrutiny following the worldwide antibiotic crisis. Scientists across the world have recognized the urgent need for new antimicrobial therapies. In this sense, antimicrobial blue light (aBL) is a promising option due to its antimicrobial properties. Although aBL can damage different cell structures, the targets responsible for bacterial inactivation have still not been completely established and require further exploration. In our study, we conducted a thorough investigation to identify the possible aBL targets and gain insights into the bactericidal effects of aBL on three relevant pathogens: Staphylococcus aureus, Escherichia coli, and Pseudomonas aeruginosa. This research not only adds new content to blue light studies but opens new perspectives to antimicrobial applications.
Keywords: endogenous chromophores; lipid peroxidation; membrane permeabilization; protein carbonylation; reactive oxygen species.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Tacconelli E, Carrara E, Savoldi A, Harbarth S, Mendelson M, Monnet DL, Pulcini C, Kahlmeter G, Kluytmans J, Carmeli Y, Ouellette M, Outterson K, Patel J, Cavaleri M, Cox EM, Houchens CR, Grayson ML, Hansen P, Singh N, Theuretzbacher U, Magrini N, Aboderin AO, Al-Abri SS, Awang Jalil N, Benzonana N, Bhattacharya S, Brink AJ, Burkert FR, Cars O, Cornaglia G, Dyar OJ, Friedrich AW, Gales AC, Gandra S, Giske CG, Goff DA, Goossens H, Gottlieb T, Guzman Blanco M, Hryniewicz W, Kattula D, Jinks T, Kanj SS, Kerr L, Kieny MP, Kim YS, Kozlov RS, Labarca J, Laxminarayan R, Leder K, WHO Pathogens Priority List Working Group , et al. 2018. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis 18:318–327. doi: 10.1016/S1473-3099(17)30753-3. - DOI - PubMed
-
- World Health Organization. 2022. WHO strategic priorities on antimicrobial resistance, Available at: https://www.who.int/publications/i/item/9789240041387. Accessed January 4, 2023.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

