Mucociliary clearance augmenting drugs block SARS-CoV-2 replication in human airway epithelial cells
- PMID: 36809189
- PMCID: PMC10042606
- DOI: 10.1152/ajplung.00285.2022
Mucociliary clearance augmenting drugs block SARS-CoV-2 replication in human airway epithelial cells
Abstract
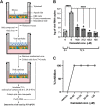
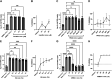
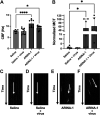
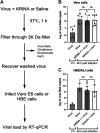
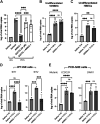
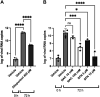
The coronavirus disease (COVID-19) pandemic, caused by SARS-CoV-2 coronavirus, is devastatingly impacting human health. A prominent component of COVID-19 is the infection and destruction of the ciliated respiratory cells, which perpetuates dissemination and disrupts protective mucociliary transport (MCT) function, an innate defense of the respiratory tract. Thus, drugs that augment MCT could improve the barrier function of the airway epithelium and reduce viral replication and, ultimately, COVID-19 outcomes. We tested five agents known to increase MCT through distinct mechanisms for activity against SARS-CoV-2 infection using a model of human respiratory epithelial cells terminally differentiated in an air/liquid interphase. Three of the five mucoactive compounds tested showed significant inhibitory activity against SARS-CoV-2 replication. An archetype mucoactive agent, ARINA-1, blocked viral replication and therefore epithelial cell injury; thus, it was further studied using biochemical, genetic, and biophysical methods to ascertain the mechanism of action via the improvement of MCT. ARINA-1 antiviral activity was dependent on enhancing the MCT cellular response, since terminal differentiation, intact ciliary expression, and motion were required for ARINA-1-mediated anti-SARS-CoV2 protection. Ultimately, we showed that the improvement of cilia movement was caused by ARINA-1-mediated regulation of the redox state of the intracellular environment, which benefited MCT. Our study indicates that intact MCT reduces SARS-CoV-2 infection, and its pharmacologic activation may be effective as an anti-COVID-19 treatment.
Keywords: SARS-CoV-2; antioxidant; cilia movement; mucoactive drug; mucociliary transport.
Conflict of interest statement
S.M.R. reports grant support from Renovion for research studies conducted through university grants/contracts and personal fees including stock options for consulting services on the design and conduct of clinical trials. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
Figures



Update of
-
Mucociliary Clearance Augmenting Drugs Block SARS-Cov-2 Replication in Human Airway Epithelial Cells.bioRxiv [Preprint]. 2023 Feb 1:2023.01.30.526308. doi: 10.1101/2023.01.30.526308. bioRxiv. 2023. Update in: Am J Physiol Lung Cell Mol Physiol. 2023 Apr 1;324(4):L493-L506. doi: 10.1152/ajplung.00285.2022. PMID: 36778446 Free PMC article. Updated. Preprint.
References
-
- Mehrabi Nejad MM, Shobeiri P, Dehghanbanadaki H, Tabary M, Aryannejad A, Haji Ghadery A, Shabani M, Moosaie F, SeyedAlinaghi SA, Rezaei N. Seroconversion following the first, second, and third dose of SARS-CoV-2 vaccines in immunocompromised population: a systematic review and meta-analysis. Virol J 19: 132, 2022. doi: 10.1186/s12985-022-01858-3. - DOI - PMC - PubMed
-
- Hoffmann M, Krüger N, Schulz S, Cossmann A, Rocha C, Kempf A, Nehlmeier I, Graichen L, Moldenhauer AS, Winkler MS, Lier M, Dopfer-Jablonka A, Jäck HM, Behrens GMN, Pöhlmann S. The Omicron variant is highly resistant against antibody-mediated neutralization: implications for control of the COVID-19 pandemic. Cell 185: 447–456.e11, 2022. doi: 10.1016/J.CELL.2021.12.032. - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

