Health worker compliance with severe malaria treatment guidelines in the context of implementing pre-referral rectal artesunate in the Democratic Republic of the Congo, Nigeria, and Uganda: An operational study
- PMID: 36809247
- PMCID: PMC9990943
- DOI: 10.1371/journal.pmed.1004189
Health worker compliance with severe malaria treatment guidelines in the context of implementing pre-referral rectal artesunate in the Democratic Republic of the Congo, Nigeria, and Uganda: An operational study
Erratum in
-
Correction: Health worker compliance with severe malaria treatment guidelines in the context of implementing pre-referral rectal artesunate in the Democratic Republic of the Congo, Nigeria, and Uganda: An operational study.PLoS Med. 2023 Apr 26;20(4):e1004234. doi: 10.1371/journal.pmed.1004234. eCollection 2023 Apr. PLoS Med. 2023. PMID: 37099752 Free PMC article.
Abstract
Background: For a full treatment course of severe malaria, community-administered pre-referral rectal artesunate (RAS) should be completed by post-referral treatment consisting of an injectable antimalarial and oral artemisinin-based combination therapy (ACT). This study aimed to assess compliance with this treatment recommendation in children under 5 years.
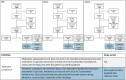
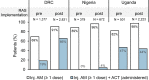
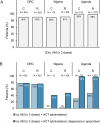
Methods and findings: This observational study accompanied the implementation of RAS in the Democratic Republic of the Congo (DRC), Nigeria, and Uganda between 2018 and 2020. Antimalarial treatment was assessed during admission in included referral health facilities (RHFs) in children under 5 with a diagnosis of severe malaria. Children were either referred from a community-based provider or directly attending the RHF. RHF data of 7,983 children was analysed for appropriateness of antimalarials; a subsample of 3,449 children was assessed additionally for dosage and method of ACT provision (treatment compliance). A parenteral antimalarial and an ACT were administered to 2.7% (28/1,051) of admitted children in Nigeria, 44.5% (1,211/2,724) in Uganda, and 50.3% (2,117/4,208) in DRC. Children receiving RAS from a community-based provider were more likely to be administered post-referral medication according to the guidelines in DRC (adjusted odds ratio (aOR) = 2.13, 95% CI 1.55 to 2.92, P < 0.001), but less likely in Uganda (aOR = 0.37, 95% CI 0.14 to 0.96, P = 0.04) adjusting for patient, provider, caregiver, and other contextual factors. While in DRC, inpatient ACT administration was common, ACTs were often prescribed at discharge in Nigeria (54.4%, 229/421) and Uganda (53.0%, 715/1,349). Study limitations include the unfeasibility to independently confirm the diagnosis of severe malaria due to the observational nature of the study.
Conclusions: Directly observed treatment was often incomplete, bearing a high risk for partial parasite clearance and disease recrudescence. Parenteral artesunate not followed up with oral ACT constitutes an artemisinin monotherapy and may favour the selection of resistant parasites. In connection with the finding that pre-referral RAS had no beneficial effect on child survival in the 3 study countries, concerns about an effective continuum of care for children with severe malaria seem justified. Stricter compliance with the WHO severe malaria treatment guidelines is critical to effectively manage this disease and further reduce child mortality.
Trial registration: ClinicalTrials.gov (NCT03568344).
Copyright: © 2023 Signorell et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Mousa A, Al-Taiar A, Anstey NM, Badaut C, Barber BE, Bassat Q, et al. The impact of delayed treatment of uncomplicated P. falciparum malaria on progression to severe malaria: A systematic review and a pooled multicentre individual-patient meta-analysis. PLoS Med. 2020;17(10):e1003359. doi: 10.1371/journal.pmed.1003359 PubMed Central PMCID: PMC7571702. - DOI - PMC - PubMed
-
- World Health Organization, Global Malaria Programme. Guidelines for malaria. Geneva. 2022.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

