Conditional ablation of heparan sulfate expression in stromal fibroblasts promotes tumor growth in vivo
- PMID: 36809261
- PMCID: PMC9942975
- DOI: 10.1371/journal.pone.0281820
Conditional ablation of heparan sulfate expression in stromal fibroblasts promotes tumor growth in vivo
Abstract
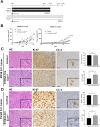
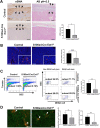
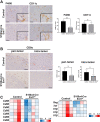
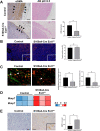
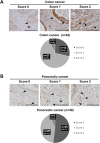
Heparan sulfate (HS) is a glycocalyx component present in the extracellular matrix and cell-surface HS proteoglycans (HSPGs). Although HSPGs are known to play functional roles in multiple aspects of tumor development and progression, the effect of HS expression in the tumor stroma on tumor growth in vivo remains unclear. We conditionally deleted Ext1, which encodes a glycosyltransferase essential for the biosynthesis of HS chains, using S100a4-Cre (S100a4-Cre; Ext1f/f) to investigate the role of HS in cancer-associated fibroblasts, which is the main component of the tumor microenvironment. Subcutaneous transplantation experiments with murine MC38 colon cancer and Pan02 pancreatic cancer cells demonstrated substantially larger subcutaneous tumors in S100a4-Cre; Ext1f/f mice. Additionally, the number of myofibroblasts observed in MC38 and Pan02 subcutaneous tumors of S100a4-Cre; Ext1f/f mice decreased. Furthermore, the number of intratumoral macrophages decreased in MC38 subcutaneous tumors in S100a4-Cre; Ext1f/f mice. Finally, the expression of matrix metalloproteinase-7 (MMP-7) markedly increased in Pan02 subcutaneous tumors in S100a4-Cre; Ext1f/f mice, suggesting that it may contribute to rapid growth. Therefore, our study demonstrates that the tumor microenvironment with HS-reduced fibroblasts provides a favorable environment for tumor growth by affecting the function and properties of cancer-associated fibroblasts, macrophages, and cancer cells.
Copyright: © 2023 Niwa et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

