Mapping the metabolic reprogramming induced by sodium-glucose cotransporter 2 inhibition
- PMID: 36809274
- PMCID: PMC10132155
- DOI: 10.1172/jci.insight.164296
Mapping the metabolic reprogramming induced by sodium-glucose cotransporter 2 inhibition
Abstract
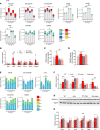
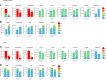
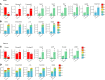
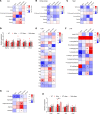
Diabetes is associated with increased risk for kidney disease, heart failure, and mortality. Sodium-glucose cotransporter 2 inhibitors (SGLT2i) prevent these adverse outcomes; however, the mechanisms involved are not clear. We generated a roadmap of the metabolic alterations that occur in different organs in diabetes and in response to SGLT2i. In vivo metabolic labeling with 13C-glucose in normoglycemic and diabetic mice treated with or without dapagliflozin, followed by metabolomics and metabolic flux analyses, showed that, in diabetes, glycolysis and glucose oxidation are impaired in the kidney, liver, and heart. Treatment with dapagliflozin failed to rescue glycolysis. SGLT2 inhibition increased glucose oxidation in all organs; in the kidney, this was associated with modulation of the redox state. Diabetes was associated with altered methionine cycle metabolism, evident by decreased betaine and methionine levels, whereas treatment with SGLT2i increased hepatic betaine along with decreased homocysteine levels. mTORC1 activity was inhibited by SGLT2i along with stimulation of AMPK in both normoglycemic and diabetic animals, possibly explaining the protective effects against kidney, liver, and heart diseases. Collectively, our findings suggest that SGLT2i induces metabolic reprogramming orchestrated by AMPK-mTORC1 signaling with common and distinct effects in various tissues, with implications for diabetes and aging.
Keywords: Diabetes; Glucose metabolism; Metabolism; Signal transduction; Therapeutics.
Conflict of interest statement
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

