Neurons sensitive to non-celestial polarized light in the brain of the desert locust
- PMID: 36809566
- PMCID: PMC10643347
- DOI: 10.1007/s00359-023-01618-w
Neurons sensitive to non-celestial polarized light in the brain of the desert locust
Abstract
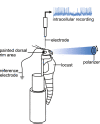
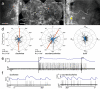
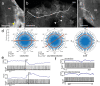
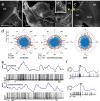
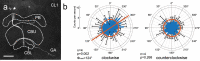
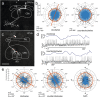
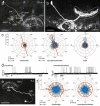
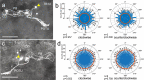
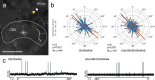
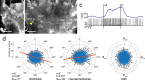
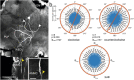
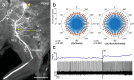
Owing to alignment of rhodopsin in microvillar photoreceptors, insects are sensitive to the oscillation plane of polarized light. This property is used by many species to navigate with respect to the polarization pattern of light from the blue sky. In addition, the polarization angle of light reflected from shiny surfaces such as bodies of water, animal skin, leaves, or other objects can enhance contrast and visibility. Whereas photoreceptors and central mechanisms involved in celestial polarization vision have been investigated in great detail, little is known about peripheral and central mechanisms of sensing the polarization angle of light reflected from objects and surfaces. Desert locusts, like other insects, use a polarization-dependent sky compass for navigation but are also sensitive to polarization angles from horizontal directions. In order to further analyze the processing of polarized light reflected from objects or water surfaces, we tested the sensitivity of brain interneurons to the angle of polarized blue light presented from ventral direction in locusts that had their dorsal eye regions painted black. Neurons encountered interconnect the optic lobes, invade the central body, or send descending axons to the ventral nerve cord but are not part of the polarization vision pathway involved in sky-compass coding.
Keywords: Central complex; Desert locust; Intracellular recordings; Non-celestial polarization vision; Sky compass coding.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Baader A, Schäfer M, Rowell CHF. The perception of the visual flow field by flying locusts: a behavioural and neuronal analysis. J Exp Biol. 1992;165:137–160. doi: 10.1242/jeb.165.1.137. - DOI
-
- Batschelet E. Circular statistics in biology. London: Academic; 1981.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

