Adrenal insufficiency in thyroid cancer patients treated with tyrosine kinase inhibitors and detected by ACTH stimulation test
- PMID: 36809657
- PMCID: PMC10348921
- DOI: 10.1007/s40618-023-02025-3
Adrenal insufficiency in thyroid cancer patients treated with tyrosine kinase inhibitors and detected by ACTH stimulation test
Abstract
Purpose: Advanced thyroid cancer patients treated with tyrosine kinase inhibitors (TKI) can develop several adverse events (AEs), including adrenal insufficiency (AI).
Methods: We studied 55 patients treated with TKI for radioiodine-refractory or medullary thyroid cancer. The adrenal function was evaluated during follow-up by performing serum basal ACTH, and basal and ACTH-stimulated cortisol.
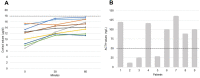
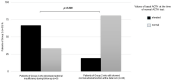
Results: Twenty-nine/55 (52.7%) patients developed subclinical AI during TKI treatment as demonstrated by a blunted cortisol response to ACTH stimulation. All cases showed normal values of serum sodium, potassium and blood pressure. All patients were immediately treated, and none showed an overt AI. Cases with AI were all negative for adrenal antibodies and did not show any adrenal gland alteration. Other causes of AI were excluded. The onset time of the AI, as measured in the subgroup with a first negative ACTH test, was < 12 months in 5/9 (55.6%), between 12 and 36 months in 2/9 (22.2%) and > 36 months in 2/9 (22.2%) cases. In our series, the only prognostic factor of AI was the elevated, although moderate, basal level of ACTH when the basal and stimulated cortisol were still normal. The glucocorticoid therapy improved fatigue in most patients.
Conclusions: Subclinical AI can be developed in > 50% of advanced thyroid cancer patients treated with TKI. This AE can develop in a wide period ranging from < 12 to > 36 months. For this reason, AI must be looked for throughout the follow-up to be early recognized and treated. A periodic ACTH stimulation test, every 6-8 months, can be helpful.
Keywords: ACTH; Adrenal insufficiency; Adverse event; Cortisol; Thyroid cancer; Tyrosine kinase inhibitors.
© 2023. The Author(s).
Conflict of interest statement
R.E. is a consultant for EISAI, IPSEN, Loxo and Bayer; the present study was not conditioned by this activity. The other authors have nothing to disclose.
Figures
References
-
- Matrone A, Valerio L, Pieruzzi L, Giani C, Cappagli V, Lorusso L, Agate L, Puleo L, Viola D, Bottici V, Del Re M, Molinaro E, Danesi R, Elisei R. Protein kinase inhibitors for the treatment of advanced and progressive radiorefractory thyroid tumors: from the clinical trials to the real life. Best Pract Res Clin Endocrinol Metab. 2017;31:319–334. doi: 10.1016/j.beem.2017.06.001. - DOI - PubMed
-
- Schlumberger M, Tahara M, Wirth LJ, Robinson B, Brose MS, Elisei R, Habra MA, Newbold K, Shah MH, Hoff AO, Gianoukakis AG, Kiyota N, Taylor MH, Kim SB, Krzyzanowska MK, Dutcus CE, de las Heras B, Zhu J, Sherman SI. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. New Engl J Med. 2015;372:621–630. doi: 10.1056/NEJMoa1406470. - DOI - PubMed
-
- Brose MS, Nutting CM, Jarzab B, Elisei R, Siena S, Bastholt L, de la Fouchardiere C, Pacini F, Paschke R, Shong YK, Sherman SI, Smit JW, Chung J, Kappeler C, Pena C, Molnar I, Schlumberger MJ, Investigators D Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet. 2014;384:319–328. doi: 10.1016/S0140-6736(14)60421-9. - DOI - PMC - PubMed
-
- Wells SA, Jr, Robinson BG, Gagel RF, Dralle H, Fagin JA, Santoro M, Baudin E, Elisei R, Jarzab B, Vasselli JR, Read J, Langmuir P, Ryan AJ, Schlumberger MJ. Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind phase III trial. J Clin Oncol. 2012;30:134–141. doi: 10.1200/JCO.2011.35.5040. - DOI - PMC - PubMed
-
- Elisei R, Schlumberger MJ, Muller SP, Schoffski P, Brose MS, Shah MH, Licitra L, Jarzab B, Medvedev V, Kreissl MC, Niederle B, Cohen EE, Wirth LJ, Ali H, Hessel C, Yaron Y, Ball D, Nelkin B, Sherman SI. Cabozantinib in progressive medullary thyroid cancer. J Clin Oncol. 2013;31:3639–3646. doi: 10.1200/JCO.2012.48.4659. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

