Physioxia improves the selectivity of hematopoietic stem cell expansion cultures
- PMID: 36809781
- PMCID: PMC10345863
- DOI: 10.1182/bloodadvances.2023009668
Physioxia improves the selectivity of hematopoietic stem cell expansion cultures
Abstract
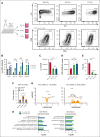
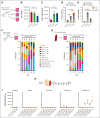
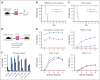
Hematopoietic stem cells (HSCs) are a rare type of hematopoietic cell that can entirely reconstitute the blood and immune system after transplantation. Allogeneic HSC transplantation (HSCT) is used clinically as a curative therapy for a range of hematolymphoid diseases; however, it remains a high-risk therapy because of its potential side effects, including poor graft function and graft-versus-host disease (GVHD). Ex vivo HSC expansion has been suggested as an approach to improve hematopoietic reconstitution in low-cell dose grafts. Here, we demonstrate that the selectivity of polyvinyl alcohol (PVA)-based mouse HSC cultures can be improved using physioxic culture conditions. Single-cell transcriptomic analysis helped confirm the inhibition of lineage-committed progenitor cells in physioxic cultures. Long-term physioxic expansion also afforded culture-based ex vivo HSC selection from whole bone marrow, spleen, and embryonic tissues. Furthermore, we provide evidence that HSC-selective ex vivo cultures deplete GVHD-causing T cells and that this approach can be combined with genotoxic-free antibody-based conditioning HSCT approaches. Our results offer a simple approach to improve PVA-based HSC cultures and the underlying molecular phenotype, and highlight the potential translational implications of selective HSC expansion systems for allogeneic HSCT.
© 2023 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: H.N. is a cofounder and shareholder in Celaid, Megakaryon and Century Therapeutics. A.C.W. is a consultant for Graphite Bio and ImmuneBridge. A.C. discloses financial interests in the following entities working in the rare genetic disease space: Beam Therapeutics, Decibel Therapeutics, Editas Medicine, Global Blood Therapeutics, GV, Lyrik Pharma, Magenta Therapeutics, and Spotlight Therapeutics. IK is currently employed by Xap Therapeutics. The remaining authors declare no competing financial interests.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

