Liquid biopsy at the frontier in renal cell carcinoma: recent analysis of techniques and clinical application
- PMID: 36810071
- PMCID: PMC9942319
- DOI: 10.1186/s12943-023-01745-7
Liquid biopsy at the frontier in renal cell carcinoma: recent analysis of techniques and clinical application
Abstract
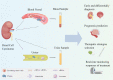
Renal cell carcinoma (RCC) is a major pathological type of kidney cancer and is one of the most common malignancies worldwide. The unremarkable symptoms of early stages, proneness to postoperative metastasis or recurrence, and low sensitivity to radiotherapy and chemotherapy pose a challenge for the diagnosis and treatment of RCC. Liquid biopsy is an emerging test that measures patient biomarkers, including circulating tumor cells, cell-free DNA/cell-free tumor DNA, cell-free RNA, exosomes, and tumor-derived metabolites and proteins. Owing to its non-invasiveness, liquid biopsy enables continuous and real-time collection of patient information for diagnosis, prognostic assessment, treatment monitoring, and response evaluation. Therefore, the selection of appropriate biomarkers for liquid biopsy is crucial for identifying high-risk patients, developing personalized therapeutic plans, and practicing precision medicine. In recent years, owing to the rapid development and iteration of extraction and analysis technologies, liquid biopsy has emerged as a low cost, high efficiency, and high accuracy clinical detection method. Here, we comprehensively review liquid biopsy components and their clinical applications over the past 5 years. Additionally, we discuss its limitations and predict its future prospects.
Keywords: Cell-free tumor DNA; Circulating tumor cells; Diagnosis; Liquid biopsy; Prognosis; Renal cell carcinoma; Treatment monitoring.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

