Fluid balance control in critically ill patients: results from POINCARE-2 stepped wedge cluster-randomized trial
- PMID: 36810101
- PMCID: PMC9945675
- DOI: 10.1186/s13054-023-04357-1
Fluid balance control in critically ill patients: results from POINCARE-2 stepped wedge cluster-randomized trial
Abstract
Background: In critically ill patients, positive fluid balance is associated with excessive mortality. The POINCARE-2 trial aimed to assess the effectiveness of a fluid balance control strategy on mortality in critically ill patients.
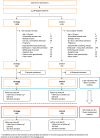
Methods: POINCARE-2 was a stepped wedge cluster open-label randomized controlled trial. We recruited critically ill patients in twelve volunteering intensive care units from nine French hospitals. Eligible patients were ≥ 18 years old, under mechanical ventilation, admitted to one of the 12 recruiting units for > 48 and ≤ 72 h, and had an expected length of stay after inclusion > 24 h. Recruitment started on May 2016 and ended on May 2019. Of 10,272 patients screened, 1361 met the inclusion criteria and 1353 completed follow-up. The POINCARE-2 strategy consisted of a daily weight-driven restriction of fluid intake, diuretics administration, and ultrafiltration in case of renal replacement therapy between Day 2 and Day 14 after admission. The primary outcome was 60-day all-cause mortality. We considered intention-to-treat analyses in cluster-randomized analyses (CRA) and in randomized before-and-after analyses (RBAA).
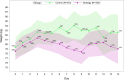
Results: A total of 433 (643) patients in the strategy group and 472 (718) in the control group were included in the CRA (RBAA). In the CRA, mean (SD) age was 63.7 (14.1) versus 65.7 (14.3) years, and mean (SD) weight at admission was 78.5 (20.0) versus 79.4 (23.5) kg. A total of 129 (160) patients died in the strategy (control) group. Sixty-day mortality did not differ between groups [30.5%, 95% confidence interval (CI) 26.2-34.8 vs. 33.9%, 95% CI 29.6-38.2, p = 0.26]. Among safety outcomes, only hypernatremia was more frequent in the strategy group (5.3% vs. 2.3%, p = 0.01). The RBAA led to similar results.
Conclusion: The POINCARE-2 conservative strategy did not reduce mortality in critically ill patients. However, due to open-label and stepped wedge design, intention-to-treat analyses might not reflect actual exposure to this strategy, and further analyses might be required before completely discarding it. Trial registration POINCARE-2 trial was registered at ClinicalTrials.gov (NCT02765009). Registered 29 April 2016.
Keywords: Clinical trial; Complex intervention; Critical care; Water-electrolyte balance.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

