Dimethyl itaconate ameliorates cognitive impairment induced by a high-fat diet via the gut-brain axis in mice
- PMID: 36810115
- PMCID: PMC9942412
- DOI: 10.1186/s40168-023-01471-8
Dimethyl itaconate ameliorates cognitive impairment induced by a high-fat diet via the gut-brain axis in mice
Erratum in
-
Correction: Dimethyl itaconate ameliorates cognitive impairment induced by a high-fat diet via the gut-brain axis in mice.Microbiome. 2023 Mar 20;11(1):55. doi: 10.1186/s40168-023-01515-z. Microbiome. 2023. PMID: 36941705 Free PMC article. No abstract available.
Abstract
Background: Gut homeostasis, including intestinal immunity and microbiome, is essential for cognitive function via the gut-brain axis. This axis is altered in high-fat diet (HFD)-induced cognitive impairment and is closely associated with neurodegenerative diseases. Dimethyl itaconate (DI) is an itaconate derivative and has recently attracted extensive interest due to its anti-inflammatory effect. This study investigated whether intraperitoneal administration of DI improves the gut-brain axis and prevents cognitive deficits in HF diet-fed mice.
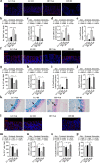
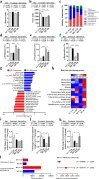
Results: DI effectively attenuated HFD-induced cognitive decline in behavioral tests of object location, novel object recognition, and nesting building, concurrent with the improvement of hippocampal RNA transcription profiles of genes associated with cognition and synaptic plasticity. In agreement, DI reduced the damage of synaptic ultrastructure and deficit of proteins (BDNF, SYN, and PSD95), the microglial activation, and neuroinflammation in the HFD-fed mice. In the colon, DI significantly lowered macrophage infiltration and the expression of pro-inflammatory cytokines (TNF-α, IL-1β, IL-6) in mice on the HF diet, while upregulating the expression of immune homeostasis-related cytokines (IL-22, IL-23) and antimicrobial peptide Reg3γ. Moreover, DI alleviated HFD-induced gut barrier impairments, including elevation of colonic mucus thickness and expression of tight junction proteins (zonula occludens-1, occludin). Notably, HFD-induced microbiome alteration was improved by DI supplementation, characterized by the increase of propionate- and butyrate-producing bacteria. Correspondingly, DI increased the levels of propionate and butyrate in the serum of HFD mice. Intriguingly, fecal microbiome transplantation from DI-treated HF mice facilitated cognitive variables compared with HF mice, including higher cognitive indexes in behavior tests and optimization of hippocampal synaptic ultrastructure. These results highlight the gut microbiota is necessary for the effects of DI in improving cognitive impairment.
Conclusions: The present study provides the first evidence that DI improves cognition and brain function with significant beneficial effects via the gut-brain axis, suggesting that DI may serve as a novel drug for treating obesity-associated neurodegenerative diseases. Video Abstract.
Keywords: Cognition; Gut microbiome; Gut-brain axis; Itaconate; Microglia; Obesity.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

