Interaction between Theta Phase and Spike Timing-Dependent Plasticity Simulates Theta-Induced Memory Effects
- PMID: 36810147
- PMCID: PMC10012328
- DOI: 10.1523/ENEURO.0333-22.2023
Interaction between Theta Phase and Spike Timing-Dependent Plasticity Simulates Theta-Induced Memory Effects
Abstract
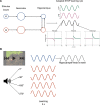
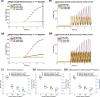
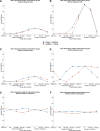
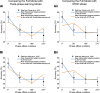
Rodent studies suggest that spike timing relative to hippocampal theta activity determines whether potentiation or depression of synapses arise. Such changes also depend on spike timing between presynaptic and postsynaptic neurons, known as spike timing-dependent plasticity (STDP). STDP, together with theta phase-dependent learning, has inspired several computational models of learning and memory. However, evidence to elucidate how these mechanisms directly link to human episodic memory is lacking. In a computational model, we modulate long-term potentiation (LTP) and long-term depression (LTD) of STDP, by opposing phases of a simulated theta rhythm. We fit parameters to a hippocampal cell culture study in which LTP and LTD were observed to occur in opposing phases of a theta rhythm. Further, we modulated two inputs by cosine waves with 0° and asynchronous phase offsets and replicate key findings in human episodic memory. Learning advantage was found for the in-phase condition, compared with the out-of-phase conditions, and was specific to theta-modulated inputs. Importantly, simulations with and without each mechanism suggest that both STDP and theta phase-dependent plasticity are necessary to replicate the findings. Together, the results indicate a role for circuit-level mechanisms, which bridge the gap between slice preparation studies and human memory.
Keywords: STDP; episodic memory; theta oscillations.
Copyright © 2023 Wang et al.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



Similar articles
-
Hebbian Spike-Timing Dependent Plasticity at the Cerebellar Input Stage.J Neurosci. 2017 Mar 15;37(11):2809-2823. doi: 10.1523/JNEUROSCI.2079-16.2016. Epub 2017 Feb 10. J Neurosci. 2017. PMID: 28188217 Free PMC article.
-
A Voltage-Based STDP Rule Combined with Fast BCM-Like Metaplasticity Accounts for LTP and Concurrent "Heterosynaptic" LTD in the Dentate Gyrus In Vivo.PLoS Comput Biol. 2015 Nov 6;11(11):e1004588. doi: 10.1371/journal.pcbi.1004588. eCollection 2015 Nov. PLoS Comput Biol. 2015. PMID: 26544038 Free PMC article.
-
Long-term population spike-timing-dependent plasticity promotes synaptic tagging but not cross-tagging in rat hippocampal area CA1.Proc Natl Acad Sci U S A. 2019 Mar 19;116(12):5737-5746. doi: 10.1073/pnas.1817643116. Epub 2019 Feb 28. Proc Natl Acad Sci U S A. 2019. PMID: 30819889 Free PMC article.
-
The spike-timing dependence of plasticity.Neuron. 2012 Aug 23;75(4):556-71. doi: 10.1016/j.neuron.2012.08.001. Neuron. 2012. PMID: 22920249 Free PMC article. Review.
-
Messages in spike timing-dependent plasticity: pros and cons.Chin J Physiol. 2010 Dec 31;53(6):359-63. doi: 10.4077/cjp.2010.amm035. Chin J Physiol. 2010. PMID: 21793347 Review.
Cited by
-
Neocortical and Hippocampal Theta Oscillations Track Audiovisual Integration and Replay of Speech Memories.J Neurosci. 2025 May 21;45(21):e1797242025. doi: 10.1523/JNEUROSCI.1797-24.2025. J Neurosci. 2025. PMID: 40389299 Free PMC article.
-
Does theta synchronicity of sensory information enhance associative memory? Replicating the theta-induced memory effect.Brain Neurosci Adv. 2024 May 24;8:23982128241255798. doi: 10.1177/23982128241255798. eCollection 2024 Jan-Dec. Brain Neurosci Adv. 2024. PMID: 38800359 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous
