Two-year outcomes following a randomised platelet transfusion trial in preterm infants
- PMID: 36810309
- PMCID: PMC10447411
- DOI: 10.1136/archdischild-2022-324915
Two-year outcomes following a randomised platelet transfusion trial in preterm infants
Abstract
Objective: Assess mortality and neurodevelopmental outcomes at 2 years of corrected age in children who participated in the PlaNeT-2/MATISSE (Platelets for Neonatal Transfusion - 2/Management of Thrombocytopenia in Special Subgroup) study, which reported that a higher platelet transfusion threshold was associated with significantly increased mortality or major bleeding compared to a lower one.
Design: Randomised clinical trial, enrolling from June 2011 to August 2017. Follow-up was complete by January 2020. Caregivers were not blinded; however, outcome assessors were blinded to treatment group.
Setting: 43 level II/III/IV neonatal intensive care units (NICUs) across UK, Netherlands and Ireland.
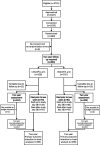
Patients: 660 infants born at less than 34 weeks' gestation with platelet counts less than 50×109/L.
Interventions: Infants were randomised to undergo a platelet transfusion at platelet count thresholds of 50×109/L (higher threshold group) or 25×109/L (lower threshold group).
Main outcomes measures: Our prespecified long-term follow-up outcome was a composite of death or neurodevelopmental impairment (developmental delay, cerebral palsy, seizure disorder, profound hearing or vision loss) at 2 years of corrected age.
Results: Follow-up data were available for 601 of 653 (92%) eligible participants. Of the 296 infants assigned to the higher threshold group, 147 (50%) died or survived with neurodevelopmental impairment, as compared with 120 (39%) of 305 infants assigned to the lower threshold group (OR 1.54, 95% CI 1.09 to 2.17, p=0.017).
Conclusions: Infants randomised to a higher platelet transfusion threshold of 50×109/L compared with 25×109/L had a higher rate of death or significant neurodevelopmental impairment at a corrected age of 2 years. This further supports evidence of harm caused by high prophylactic platelet transfusion thresholds in preterm infants.
Trial registration number: ISRCTN87736839.
Keywords: Child Development; Intensive Care Units, Neonatal; Neonatology.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
MeSH terms
LinkOut - more resources
Full Text Sources
Medical