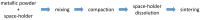Tantalum as Trabecular Metal for Endosseous Implantable Applications
- PMID: 36810380
- PMCID: PMC9944482
- DOI: 10.3390/biomimetics8010049
Tantalum as Trabecular Metal for Endosseous Implantable Applications
Abstract
During the last 20 years, tantalum has known ever wider applications for the production of endosseous implantable devices in the orthopedic and dental fields. Its excellent performances are due to its capacity to stimulate new bone formation, thus improving implant integration and stable fixation. Tantalum's mechanical features can be mainly adjusted by controlling its porosity thanks to a number of versatile fabrication techniques, which allow obtaining an elastic modulus similar to that of bone tissue, thus limiting the stress-shielding effect. The present paper aims at reviewing the characteristics of tantalum as a solid and porous (trabecular) metal, with specific regard to biocompatibility and bioactivity. Principal fabrication methods and major applications are described. Moreover, the osteogenic features of porous tantalum are presented to testify its regenerative potential. It can be concluded that tantalum, especially as a porous metal, clearly possesses many advantageous characteristics for endosseous applications but it presently lacks the consolidated clinical experience of other metals such as titanium.
Keywords: endosseous devices; osteointegration; porous metal; tantalum; trabecular metal.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Registro Italiano ArtroProtesi . Annual Report. RIAP; Rome, Italy: 2020.
-
- [(accessed on 1 August 2022)]. Available online: https://www.researchandmarkets.com/reports/4599561/orthopedic-devices-ma....
-
- [(accessed on 1 August 2022)]. Available online: https://www.todaysmedicaldevelopments.com/article/medical-device-orthope...
-
- Adamovic D., Ristic B., Zivic F. Review of Existing Biomaterials—Method of Material Selection for Specific Applications in Orthopedics. In: Zivic F., Affatato S., Trajanovic M., Schnabelrauch M., Grujovic N., Choy K., editors. Biomaterials in Clinical Practice. Springer; Cham, Switzerland: 2018. pp. 47–99. - DOI
Publication types
LinkOut - more resources
Full Text Sources