Migraine Disability Improvement during Treatment with Galcanezumab in Patients with Chronic and High Frequency Episodic Migraine
- PMID: 36810472
- PMCID: PMC9944445
- DOI: 10.3390/neurolint15010017
Migraine Disability Improvement during Treatment with Galcanezumab in Patients with Chronic and High Frequency Episodic Migraine
Abstract
Background: The aim of the present study was to assess the migraine outcome, in particular migraine disability, in chronic (CM) and high frequency episodic migraine (HFEM) patients in treatment with galcanezumab.
Methods: The present study was conducted at the Headache Centre of Spedali Civili of Brescia. Patients were treated with galcanezumab 120 mg monthly. Clinical and demographical information were collected at the baseline (T0). Data about outcome, analgesics consumption and disability (MIDAS and HIT-6 scores) were collected quarterly.
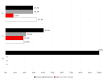
Results: Fifty-four consecutive patients were enrolled. Thirty-seven patients had a diagnosis of CM, 17 of HFEM. During treatment, patients reported a significant reduction in terms of mean headache/migraine days (p < 0.001), the attacks' pain intensity (p = 0.001) and monthly consumed analgesics (p < 0.001). The MIDAS and HIT-6 scores also documented a significant improvement (p < 0.001). At the baseline, all patients documented a severe degree of disability (MIDAS score ≥ 21). Following six months of treatment, only 29.2% of patients still documented a MIDAS score ≥ 21, with one third of patients documenting little or no disability. A > 50% MIDAS reduction, compared to baseline, was observed in up to 94.6% of patients, following the first three months of treatment. A similar outcome was found for HIT-6 scores. A significant positive correlation was found between headache days and MIDAS at T3 and T6 (T6 > T3), but not baseline.
Discussion: Monthly prophylactic treatment with galcanezumab was found to be effective in both CM and HFEM, especially in reducing migraine burden and disability.
Keywords: CGRP; HIT-6; MIDAS; burden; disability; galcanezumab; migraine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Headache Classification Committee of the International Headache Society The International Classification of Headache Disorders. Cephalalgia. 2018;38:1–211. - PubMed
-
- Feigin V.L., Nichols E., Alam T., Bannick M.S., Beghi E., Blake N., Culpepper W.J., Dorsey E.R., Elbaz A., Ellenbogen R.G., et al. Global, regional, and national burden of neurological disorders, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:459–480. doi: 10.1016/S1474-4422(18)30499-X. - DOI - PMC - PubMed
-
- Stovner L.J., Nichols E., Steiner T.J., Abd-Allah F., Abdelalim A., Al-Raddadi R.M., Ansha M.G., Barac A., Bensenor I., Doan L.P., et al. Global, regional, and national burden of migraine and tension-type headache, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018;17:954–976. doi: 10.1016/S1474-4422(18)30322-3. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

