Thiotepa, Busulfan, Cyclophosphamide: Effective but Toxic Conditioning Regimen Prior to Autologous Hematopoietic Stem Cell Transplantation in Central Nervous System Lymphoma
- PMID: 36810481
- PMCID: PMC9944873
- DOI: 10.3390/medsci11010014
Thiotepa, Busulfan, Cyclophosphamide: Effective but Toxic Conditioning Regimen Prior to Autologous Hematopoietic Stem Cell Transplantation in Central Nervous System Lymphoma
Abstract
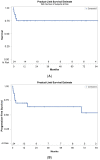
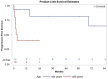
In primary central nervous system lymphoma, two-year progression-free survival rates of up to 63 percent have been reported for first-line autologous stem cell transplantation after conditioning with the thiotepa busulfan cyclophosphamide regimen. However, 11 percent of the patients died due to toxicity. Besides conventional survival, progression-free survival and treatment related mortality analyses, a competing-risk analysis was applied to our cohort of twenty-four consecutive patients with primary or secondary central nervous system lymphoma who underwent autologous stem cell transplantation after thiotepa busulfan cyclophosphamide conditioning. The two-year overall survival and progression-free survival rates were 78 percent and 65 percent, respectively. The treatment-related mortality rate was 21 percent. The competing risks analysis demonstrate that age 60 or over and the infusion of less than 4.6 × 106/kg CD34+ stem cells were significant adverse prognostic factors for overall survival. Autologous stem cell transplantation with thiotepa busulfan cyclophosphamide conditioning was associated with sustained remission and survival. Nevertheless, the intensive thiotepa busulfan cyclophosphamide conditioning regimen was highly toxic, especially in older patients. Thus, our results suggest that future studies should aim at identifying the subgroup of patients who will really benefit of the procedure and/or to reduce the toxicity of future conditioning regimen.
Keywords: adverse events; autologous hematopoietic stem cell transplantation; central nervous system lymphoma; conditioning; infections.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Haas R.L.M., Poortmans P., de Jong D., Aleman B.M.P., Dewit L.G.H., Verheij M., Hart A.A.M., van Oers M.H.J., van der Hulst M., Baars J.W., et al. High Response Rates and Lasting Remissions After Low-Dose Involved Field Radiotherapy in Indolent Lymphomas. J. Clin. Oncol. 2003;21:2474–2480. doi: 10.1200/JCO.2003.09.542. - DOI - PubMed
-
- O’Brien P.C., Roos D.E., Pratt G., Liew K.-H., Barton M.B., Poulsen M.G., Olver I.N., Trotter G.E. Combined-modality therapy for primary central nervous system lymphoma: Long-term data from a Phase II multicenter study (Trans-Tasman Radiation Oncology Group) Int. J. Radiat. Oncol. *Biol. *Phys. 2006;64:408–413. doi: 10.1016/j.ijrobp.2005.07.958. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical