Characterization of an IDH1 R132H Rabbit Monoclonal Antibody, MRQ-67, and Its Applications in the Identification of Diffuse Gliomas
- PMID: 36810519
- PMCID: PMC9944093
- DOI: 10.3390/antib12010014
Characterization of an IDH1 R132H Rabbit Monoclonal Antibody, MRQ-67, and Its Applications in the Identification of Diffuse Gliomas
Abstract
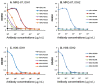
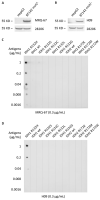
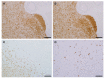
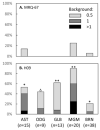
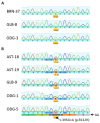
The current diagnosis of diffuse glioma involves isocitrate dehydrogenase (IDH) mutation testing. Most IDH mutant gliomas carry a G-to-A mutation at IDH1 position 395, resulting in the R132H mutant. R132H immunohistochemistry (IHC), therefore, is used to screen for the IDH1 mutation. In this study, the performance of MRQ-67, a recently generated IDH1 R132H antibody, was characterized in comparison with H09, a frequently used clone. Selective binding was demonstrated by an enzyme-linked immunosorbent assay for MRQ-67 to the R132H mutant, with an affinity higher than that for H09. By Western and dot immunoassays, MRQ-67 was found to bind specifically to the IDH1 R1322H, with a higher capacity than H09. IHC testing with MRQ-67 demonstrated a positive signal in most diffuse astrocytomas (16/22), oligodendrogliomas (9/15), and secondary glioblastomas tested (3/3), but not in primary glioblastomas (0/24). While both clones demonstrated a positive signal with similar patterns and equivalent intensities, H09 exhibited a background stain more frequently. DNA sequencing on 18 samples showed the R132H mutation in all IHC positive cases (5/5), but not in negative cases (0/13). These results demonstrate that MRQ-67 is a high-affinity antibody suitable for specific detection of the IDH1 R132H mutant by IHC and with less background as compared with H09.
Keywords: IDH1 R132H mutant; dot immunoassay; glioma; immunohistochemistry; isocitrate dehydrogenase 1; monoclonal antibody.
Conflict of interest statement
All authors are employees of MilliporeSigma, and the company provided necessary supports and resources in the study. The objectivity and authenticity of the experimental results and discussion were not affected by the company.
Figures




Similar articles
-
Generation and Performance of R132H Mutant IDH1 Rabbit Monoclonal Antibody.Antibodies (Basel). 2017 Dec 1;6(4):22. doi: 10.3390/antib6040022. Antibodies (Basel). 2017. PMID: 31548536 Free PMC article.
-
Frequent false-negative immunohistochemical staining with IDH1 (R132H)-specific H09 antibody on frozen section control slides: a potential pitfall in glioma diagnosis.Histopathology. 2019 Jan;74(2):350-354. doi: 10.1111/his.13756. Epub 2018 Dec 5. Histopathology. 2019. PMID: 30221786
-
Determining IDH-Mutational Status in Gliomas Using IDH1-R132H Antibody and Polymerase Chain Reaction.Appl Immunohistochem Mol Morphol. 2019 Nov/Dec;27(10):722-725. doi: 10.1097/PAI.0000000000000702. Appl Immunohistochem Mol Morphol. 2019. PMID: 30358614
-
Specific monoclonal antibodies against IDH1/2 mutations as diagnostic tools for gliomas.Brain Tumor Pathol. 2015 Jan;32(1):3-11. doi: 10.1007/s10014-014-0202-4. Epub 2014 Oct 17. Brain Tumor Pathol. 2015. PMID: 25324168 Review.
-
IDH mutations in older patients with diffuse astrocytic gliomas.Ann Diagn Pathol. 2020 Dec;49:151653. doi: 10.1016/j.anndiagpath.2020.151653. Epub 2020 Oct 25. Ann Diagn Pathol. 2020. PMID: 33137656 Review.
Cited by
-
Modernizing Neuro-Oncology: The Impact of Imaging, Liquid Biopsies, and AI on Diagnosis and Treatment.Int J Mol Sci. 2025 Jan 22;26(3):917. doi: 10.3390/ijms26030917. Int J Mol Sci. 2025. PMID: 39940686 Free PMC article. Review.
References
-
- Louis D.N., von Deimling A., Cavenee W.K. Diffuse astrocytic and oligodendroglial tumors—Introduction. In: Louis D.N., Ohgaki H., Wiestler O.D., Cavenee W.K., editors. WHO Classification of Tumors of the Central Nervous System. 4th ed. International Agency for Research on Cancer; Lyon, France: 2016. pp. 16–17.
-
- Louis D.N., Perry A., Reifenberger G., von Deimling A., Figarella-Branger D., Cavenee W.K., Ohgaki H., Wiestler O.D., Kleihues P., Ellison D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016;131:803–820. doi: 10.1007/s00401-016-1545-1. - DOI - PubMed
-
- Louis D.N., Perry A., Wesseling P., Brat D.J., Cree I.A., Figarella-Branger D., Hawkins C., Ng H.K., Pfister S.M., Reifenberger G., et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro Oncol. 2021;23:1231–1251. doi: 10.1093/neuonc/noab106. - DOI - PMC - PubMed
-
- Rushing E.J. WHO classification of tumors of the nervous system: Preview of the upcoming 5th edition. MEMO. 2021;14:188–191. doi: 10.1007/s12254-021-00680-x. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

