Comparative Study on MNVT of OPV Type I and III Reference Products in Different Periods
- PMID: 36810542
- PMCID: PMC9944487
- DOI: 10.3390/diseases11010028
Comparative Study on MNVT of OPV Type I and III Reference Products in Different Periods
Abstract
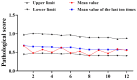
Widespread vaccination using the oral live attenuated polio vaccine (OPV) and Sabin strain inactivated vaccine (sIPV) have greatly reduced the incidence of polio worldwide. In the period post-polio, the virulence of reversion of the Sabin strain makes the use of OPV gradually becoming one of the major safety hazards. The verification and release of OPV has become the top priority. The monkey neurovirulence test (MNVT) is the gold standard for detecting whether OPV meets the criteria, which are recommended by the WHO and Chinese Pharmacopoeia. Therefore, we statistically analyzed the MNVT results of type I and III OPV at different stages: 1996-2002 and 2016-2022. The results show that the upper and lower limits and C value of the qualification standard of type I reference products in 2016-2022 have decreased compared with the corresponding scores in the 1996-2002 period. The upper and lower limit and C value of the qualified standard of type III reference products were basically the same as the corresponding scores in the 1996-2002. We also found significant differences in the pathogenicity of the type I and III in the cervical spine and brain, with the decreasing trend in the diffusion index of the type I and type III in the cervical spine and brain. Finally, two evaluation criteria were used to judge the OPV test vaccines from 2016 to 2022. The vaccines all met the test requirements under the evaluation criteria of the above two stages. Based on the characteristics of OPV, data monitoring was one of the most intuitive methods to judge changes in virulence.
Keywords: MNVT; OPV; neurovirulence; reference product; vaccine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Mutations in Sabin 2 strain of poliovirus and stability of attenuation phenotype.Virology. 1999 May 25;258(1):152-60. doi: 10.1006/viro.1999.9718. Virology. 1999. PMID: 10329577
-
Surveillance of immunity acquired from poliovirus immunization including vaccination with the Sabin strain-derived inactivated vaccine.Hum Vaccin Immunother. 2019;15(5):1154-1159. doi: 10.1080/21645515.2019.1572408. Epub 2019 Mar 11. Hum Vaccin Immunother. 2019. PMID: 30676843 Free PMC article.
-
Differences in Antigenic Structure of Inactivated Polio Vaccines Made From Sabin Live-Attenuated and Wild-Type Poliovirus Strains: Impact on Vaccine Potency Assays.J Infect Dis. 2020 Feb 3;221(4):544-552. doi: 10.1093/infdis/jiz076. J Infect Dis. 2020. PMID: 30788503
-
Some aspects of the monkey neurovirulence test used for the assessment of oral poliovirus vaccines.Dev Biol Stand. 1993;78:61-70. Dev Biol Stand. 1993. PMID: 8388832 Review.
-
Polio endgame: the global introduction of inactivated polio vaccine.Expert Rev Vaccines. 2015 May;14(5):749-62. doi: 10.1586/14760584.2015.1001750. Epub 2015 Jan 19. Expert Rev Vaccines. 2015. PMID: 25597843 Review.
References
-
- Cabrerizo M. Grupo Para El Estudio de Las Infecciones Por Enterovirus Y Parechovirus GPEELIPEYP. Importancia de los enterovirus en neuropediatria: De los poliovirus a otros enterovirus [Importance of enteroviruses in neuropaediatrics: From polioviruses to other enteroviruses] Rev. Neurol. 2017;64:S35–S38. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

