The Coronavirus Disease 2019 Rebound Study: A Prospective Cohort Study to Evaluate Viral and Symptom Rebound Differences in Participants Treated With Nirmatrelvir Plus Ritonavir Versus Untreated Controls
- PMID: 36810665
- PMCID: PMC10320179
- DOI: 10.1093/cid/ciad102
The Coronavirus Disease 2019 Rebound Study: A Prospective Cohort Study to Evaluate Viral and Symptom Rebound Differences in Participants Treated With Nirmatrelvir Plus Ritonavir Versus Untreated Controls
Abstract
Background: The uptake of nirmatrelvir plus ritonavir (NPR) in patients with coronavirus disease 2019 (COVID-19) has been limited by concerns around the rebound phenomenon despite the scarcity of evidence around its epidemiology. The purpose of this study was to prospectively compare the epidemiology of rebound in NPR-treated and untreated participants with acute COVID-19 infection.
Methods: We designed a prospective, observational study in which participants who tested positive for COVID-19 and were clinically eligible for NPR were recruited to be evaluated for either viral or symptom clearance and rebound. Participants were assigned to the treatment or control group based on their decision to take NPR. Following initial diagnosis, both groups were provided 12 rapid antigen tests and asked to test on a regular schedule for 16 days and answer symptom surveys. Viral rebound based on test results and COVID-19 symptom rebound based on patient-reported symptoms were evaluated.
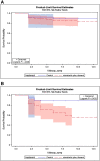
Results: Viral rebound incidence was 14.2% in the NPR treatment group (n = 127) and 9.3% in the control group (n = 43). Symptom rebound incidence was higher in the treatment group (18.9%) compared to controls (7.0%). There were no notable differences in viral rebound by age, gender, preexisting conditions, or major symptom groups during the acute phase or at the 1-month interval.
Conclusions: This preliminary report suggests that rebound after clearance of test positivity or symptom resolution is higher than previously reported. However, notably we observed a similar rate of rebound in both the NPR treatment and control groups. Large studies with diverse participants and extended follow-up are needed to better understand the rebound phenomena.
Keywords: COVID-19; COVID-19 rebound; nirmatrelvir plus ritonavir; symptom rebound; viral rebound.
© The Author(s) 2023. Published by Oxford University Press on behalf of Infectious Diseases Society of America. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Conflict of interest statement
Potential conflicts of interest. M. J. M. is Chief Science Officer of eMed (stock or stock options reported), on the board of directors for Quantum-SI, a protein sequencing company (stock or stock reported), on the scientific advisory board for ImmuneID, a phage display company, and serves on the medical advisory board for 4 Catalyzer. Additionally, M. J. M. reports receipt of consulting fees as a Medical/Science Advisor for Detect, Inc., a Medical Advisor for Quantum SI, a Public Health/Medical Advisor for LivePerson, and a Public Health Consultant for LiquiNano Tech. JBP reports an unpaid position as a Board Member at the San Diego Spine Foundation. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures


References
-
- Farley J. FDA Updates on Paxlovid for Health Care Providers. Available at: https://www.fda.gov/drugs/news-events-human-drugs/fda-updates-paxlovid-h.... Accessed 7 November 2022.
-
- Administration USFD . Coronavirus (COVID-19) Update: FDA Authorizes Additional Oral Antiviral for Treatment of COVID-19 in Certain Adults. Available at: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19.... Accessed 7 November 2022.
-
- Pfizer . Pfizer to Provide US Government with an Additional 10 Million Treatment Courses of Its Oral Therapy to Help Combat COVID-19. Available at: https://www.pfizer.com/news/press-release/press-release-detail/pfizer-pr.... Accessed 7 November 2022.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

