German translation of the PROMIS® pediatric anxiety, anger, depressive symptoms, fatigue, pain interference and peer relationships item banks
- PMID: 36810699
- PMCID: PMC9943801
- DOI: 10.1186/s41687-023-00548-0
German translation of the PROMIS® pediatric anxiety, anger, depressive symptoms, fatigue, pain interference and peer relationships item banks
Abstract
Background: The present study aimed at the translation and cross-cultural adaptation of six PROMIS® pediatric self- and proxy- item banks and short forms to universal German: anxiety (ANX), anger (ANG), depressive symptoms (DEP), Fatigue (FAT), pain interference (P) and peer relationships (PR).
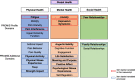
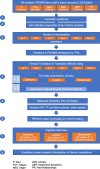
Methods: Using standardized methodology approved by the PROMIS Statistical Center and in line with recommendations of the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) PRO Translation Task Force, two translators for each German-speaking country (Germany, Austria, and Switzerland) commented on and rated the translation difficulty and provided forward translations, followed by a review and reconciliation phase. An independent translator performed back translations, which were reviewed and harmonized. The items were tested in cognitive interviews with 58 children and adolescents from Germany (16), Austria (22), and Switzerland (20) for the self-report and 42 parents and other caregivers (Germany (12), Austria (17), and Switzerland (13)) for the proxy-report.
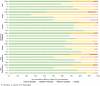
Results: Translators rated the translation difficulty of most items (95%) as easy or feasible. Pretesting showed that items of the universal German version were understood as they were intended, as only 14 out of 82 items of the self-report and 15 out of 82 items of the proxy-report versions required minor rewording. However, on average German translators rated the items more difficult to translate (M = 1.5, SD = 0.20) than the Austrian (M = 1.3, SD = 0.16) and the Swiss translators (M = 1.2, SD = 0.14) on a three-point Likert scale.
Conclusions: The translated German short forms are ready for use by researchers and clinicians ( https://www.healthmeasures.net/search-view-measures ).
Keywords: Anger; Anxiety; Depression; Fatigue; German; PROMIS; Pain; Pediatric health; Peer; Translation.
Plain language summary
A multitude of questionnaires exist, which are not comparable due to different questions or no available translations. PROMIS is an initiative, which was funded by the National Institute of Health in the US, to build better, i.e., more precise and efficient questionnaires, which can be used and compared worldwide. The PROMIS questionnaires include paper-and-pencil short forms and computerized adaptive tests. So far numerous PROMIS surveys have been created using advanced methodologies. They can be used by health care professionals to assess different aspects of health and compare the results internationally. To allow for international comparability of studies using those questionnaires, they need to be translated. This study reports the thorough translation process of the US-American PROMIS® questionnaires measuring anxiety, anger, depressive symptoms, fatigue, pain interference, and peer relationships in children and adolescents into German. The translation included researchers, children, and parents from Germany, Austria, and Switzerland to ensure that the final German version is fully and equally well understood in all of those German-speaking countries. The article describes the translation process, so that the user can understand the translations and use them in an informed way. The translated German questionnaires are ready for use by researchers and clinicians. ( https://www.healthmeasures.net/search-view-measures ).
© 2023. The Author(s).
Conflict of interest statement
The authors have no relevant financial or non-financial interests to disclose.
Figures
References
-
- Cella D, Yount S, Rothrock N, Gershon R, Cook K, Reeve B, Ader D, Fries JF, Bruce B, Rose M, PROMIS Cooperative Group (2007) The patient-reported outcomes measurement information system (PROMIS): progress of an NIH Roadmap cooperative group during its first two years. Med Care 45:S3–S1110.1097/01.mlr.0000258615.42478.55 - PMC - PubMed
-
- Forrest CB, Bevans KB, Tucker C, Riley AW, Ravens-Sieberer U, Gardner W, Pajer K. Commentary: the patient-reported outcome measurement information system (PROMIS®) for children and youth: application to pediatric psychology. J Pediatr Psychol. 2012;37:614–621. doi: 10.1093/jpepsy/jss038. - DOI - PMC - PubMed
-
- Cella D, Riley W, Stone A, Rothrock N, Reeve B, Yount S, Amtmann D, Bode R, Buysse D, Choi S. The patient-reported outcomes measurement information system (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J Clin Epidemiol. 2010;63:1179–1194. doi: 10.1016/j.jclinepi.2010.04.011. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous