Enhanced Expression of Plasminogen Activators and Inhibitor in the Healing of Tympanic Membrane Perforation in Rats
- PMID: 36810718
- PMCID: PMC10121974
- DOI: 10.1007/s10162-023-00891-5
Enhanced Expression of Plasminogen Activators and Inhibitor in the Healing of Tympanic Membrane Perforation in Rats
Abstract
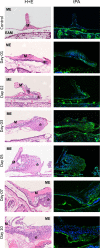
The significance of plasminogen activation during the tympanic membrane (TM) healing is known mainly from studies performed on knock-out mice. In the previous study, we reported activation of genes coding proteins of plasminogen activation and inhibition system in rat's TM perforation healing. The aim of the present study was the evaluation of protein products expressed by these genes and their tissue distribution using Western blotting and immunofluorescent method, respectively, during 10-day observation period after injury. Otomicroscopical and histological evaluation were employed to assess the healing process. The expression of urokinase plasminogen activator (uPA) and its receptor (uPAR) were significantly upregulated in the proliferation phase, with subsequent gradual attenuation during remodeling phase of healing process, when keratinocyte migration was weakening. The expression of plasminogen activator inhibitor type 1 (PAI-1) also showed the highest levels during the proliferation phase. The increase of tissue plasminogen activator (tPA) expression was observed during the whole observation period, with the highest activity during the remodeling phase. Immunofluorescence of these proteins was present mainly in migrating epithelium. Our study found that plasminogen activation (uPA, uPAR, tPA) and inhibitory (PAI-1) molecules form a well-structured regulatory system of the epithelial migration that is critical to the healing of TM after its perforation.
Keywords: Plasminogen activator inhibitor type 1; Rats; Tissue-type plasminogen activator; Tympanic membrane perforation; Urokinase-type plasminogen activator; Urokinase-type plasminogen activator receptor.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

