Phosphodiesterase 8 governs cAMP/PKA-dependent reduction of L-type calcium current in human atrial fibrillation: a novel arrhythmogenic mechanism
- PMID: 36810794
- PMCID: PMC10344654
- DOI: 10.1093/eurheartj/ehad086
Phosphodiesterase 8 governs cAMP/PKA-dependent reduction of L-type calcium current in human atrial fibrillation: a novel arrhythmogenic mechanism
Abstract
Aims: Atrial fibrillation (AF) is associated with altered cAMP/PKA signaling and an AF-promoting reduction of L-type Ca2+-current (ICa,L), the mechanisms of which are poorly understood. Cyclic-nucleotide phosphodiesterases (PDEs) degrade cAMP and regulate PKA-dependent phosphorylation of key calcium-handling proteins, including the ICa,L-carrying Cav1.2α1C subunit. The aim was to assess whether altered function of PDE type-8 (PDE8) isoforms contributes to the reduction of ICa,L in persistent (chronic) AF (cAF) patients.
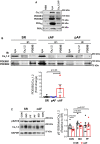
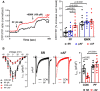
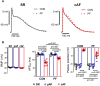
Methods and results: mRNA, protein levels, and localization of PDE8A and PDE8B isoforms were measured by RT-qPCR, western blot, co-immunoprecipitation and immunofluorescence. PDE8 function was assessed by FRET, patch-clamp and sharp-electrode recordings. PDE8A gene and protein levels were higher in paroxysmal AF (pAF) vs. sinus rhythm (SR) patients, whereas PDE8B was upregulated in cAF only. Cytosolic abundance of PDE8A was higher in atrial pAF myocytes, whereas PDE8B tended to be more abundant at the plasmalemma in cAF myocytes. In co-immunoprecipitation, only PDE8B2 showed binding to Cav1.2α1C subunit which was strongly increased in cAF. Accordingly, Cav1.2α1C showed a lower phosphorylation at Ser1928 in association with decreased ICa,L in cAF. Selective PDE8 inhibition increased Ser1928 phosphorylation of Cav1.2α1C, enhanced cAMP at the subsarcolemma and rescued the lower ICa,L in cAF, which was accompanied by a prolongation of action potential duration at 50% of repolarization.
Conclusion: Both PDE8A and PDE8B are expressed in human heart. Upregulation of PDE8B isoforms in cAF reduces ICa,L via direct interaction of PDE8B2 with the Cav1.2α1C subunit. Thus, upregulated PDE8B2 might serve as a novel molecular mechanism of the proarrhythmic reduction of ICa,L in cAF.
Keywords: Atrial fibrillation; Calcium handling; ICa,L; PDE8; Phosphodiesterases; cAMP.
© The Author(s) 2023. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
Conflict of interest The authors have no competing interests. R.B.S. reports grants from European Union Horizon 2020, the German Ministry of Research and Education, ERACOSysMed3 and personal fees from BMS/Pfizer, outside this work.
Figures


Comment in
-
Dysregulated Ca2+ cycling in atrial fibrillation.Eur Heart J. 2023 Jul 14;44(27):2495-2497. doi: 10.1093/eurheartj/ehad099. Eur Heart J. 2023. PMID: 37012620 Free PMC article.
References
-
- Voigt N, Li N, Wang Q, Wang W, Trafford AW, Abu-Taha I, et al. . Enhanced sarcoplasmic reticulum Ca2 + leak and increased na+-Ca2 + exchanger function underlie delayed afterdepolarizations in patients with chronic atrial fibrillation. Circulation 2012;125:2059–2070. 10.1161/CIRCULATIONAHA.111.067306 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

