Ocular adverse events associated with immune checkpoint inhibitors, a scoping review
- PMID: 36811715
- PMCID: PMC9947214
- DOI: 10.1186/s12348-022-00321-2
Ocular adverse events associated with immune checkpoint inhibitors, a scoping review
Abstract
Introduction: Immune checkpoint inhibitors (ICIs) have become an important part of the treatment of multiple cancers, especially for advanced melanoma and non-small cell lung cancer. Some tumors are capable of escaping immunosurveillance by stimulating checkpoints on T-cells. ICIs prevent activation of these checkpoints and thereby stimulate the immune system and indirectly the anti-tumor response. However, the use of ICIs is associated with various adverse events. Ocular side effects are rare but may have a major impact on the quality of life of the patient.
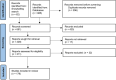
Methods: A comprehensive literature search of the medical databases Web of Science, Embase and PubMed was performed. Articles that provided a comprehensive description of a case report containing 1) cancer patient(s) treated with (a combination of) immune checkpoint inhibitors, and 2) assessed occurrence of ocular adverse events, were included. A total of 290 case reports were included.
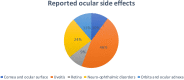
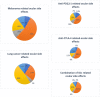
Results: Melanoma (n = 179; 61.7%) and lung cancer (n = 56; 19.3%) were the most frequent reported malignancies. The primary used ICIs were nivolumab (n = 123; 42.5%) and ipilimumab (n = 116; 40.0%). Uveitis was most the common adverse event (n = 134; 46.2%) and mainly related to melanoma. Neuro-ophthalmic disorders, including myasthenia gravis and cranial nerve disorders, were the second most common adverse events (n = 71; 24.5%), mainly related to lung cancer. Adverse events affecting the orbit and the cornea were reported in 33 (11.4%) and 30 cases (10.3%) respectively. Adverse events concerning the retina were reported in 26 cases (9.0%).
Conclusion: The aim of this paper is to provide an overview of all reported ocular adverse events related to the use of ICIs. The insights retrieved from this review might contribute to a better understanding of the underlying mechanisms of these ocular adverse events. Particularly, the difference between actual immune-related adverse events and paraneoplastic syndromes might be relevant. These findings might be of great value in establishing guidelines on how to manage ocular adverse events related to ICIs.
Keywords: Eye; Immune checkpoint inhibitors; Immune related adverse event; Paraneoplastic.
© 2023. The Author(s).
Conflict of interest statement
No conflicting relationship exists for any author.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources

