An interaction between β'-COP and the ArfGAP, Glo3, maintains post-Golgi cargo recycling
- PMID: 36811888
- PMCID: PMC9960064
- DOI: 10.1083/jcb.202008061
An interaction between β'-COP and the ArfGAP, Glo3, maintains post-Golgi cargo recycling
Abstract
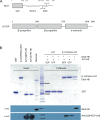
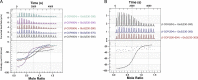
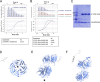
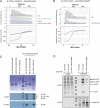
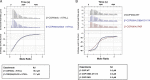
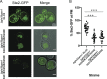
The essential COPI coat mediates retrieval of transmembrane proteins at the Golgi and endosomes following recruitment by the small GTPase, Arf1. ArfGAP proteins regulate COPI coats, but molecular details for COPI recognition by ArfGAPs remain elusive. Biochemical and biophysical data reveal how β'-COP propeller domains directly engage the yeast ArfGAP, Glo3, with a low micromolar binding affinity. Calorimetry data demonstrate that both β'-COP propeller domains are required to bind Glo3. An acidic patch on β'-COP (D437/D450) interacts with Glo3 lysine residues located within the BoCCS (binding of coatomer, cargo, and SNAREs) region. Targeted point mutations in either Glo3 BoCCS or β'-COP abrogate the interaction in vitro, and loss of the β'-COP/Glo3 interaction drives Ste2 missorting to the vacuole and aberrant Golgi morphology in budding yeast. These data suggest that cells require the β'-COP/Glo3 interaction for cargo recycling via endosomes and the TGN, where β'-COP serves as a molecular platform to coordinate binding to multiple proteins, including Glo3, Arf1, and the COPI F-subcomplex.
© 2023 Xie et al.
Conflict of interest statement
Disclosures: The authors declare no competing interests exist.
Figures







References
-
- Bhandari, A., Zheng C., Sindan N., Sindan N., Quan R., Xia E., Thapa Y., Tamang D., Wang O., Ye X., and Huang D.. 2019. COPB2 is up-regulated in breast cancer and plays a vital role in the metastasis via N-cadherin and Vimentin. J. Cell. Mol. Med. 23:5235–5245. 10.1111/jcmm.14398 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

