Association of Days Alive and Out of the Hospital After Ventricular Assist Device Implantation With Adverse Events and Quality of Life
- PMID: 36811897
- PMCID: PMC9947806
- DOI: 10.1001/jamasurg.2022.8127
Association of Days Alive and Out of the Hospital After Ventricular Assist Device Implantation With Adverse Events and Quality of Life
Abstract
Importance: There is a need to better assess the cumulative effect on morbidity and mortality in patients undergoing durable left ventricular assist device (LVAD) implantation. This study evaluates a patient-centered performance metric (days alive and out of hospital [DAOH]) for durable LVAD therapy.
Objective: To determine the incidence of percent of DAOH before and after LVAD implantation and (2) explore its association with established quality metrics (death, adverse events [AEs], quality of life).
Design, settings, and participants: This was a retrospective national cohort study of Medicare beneficiaries implanted with a durable continuous-flow LVAD between April 2012 and December 2016. The data were analyzed from December 2021 to May 2022. Follow-up was 100% complete at 1 year. Data from The Society of Thoracic Surgeons Intermacs registry were linked to Medicare claims.
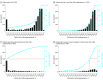
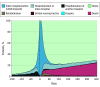
Main outcomes and measures: The number of DAOH 180 days before and 365 days after LVAD implantation and daily patient location (home, index hospital, nonindex hospital, skilled nursing facility, rehabilitation center, hospice) were calculated. Percent of DAOH was indexed to each beneficiary's pre- (percent DAOH-BF) and postimplantation (percentage of DAOH-AF) follow-up time. The cohort was stratified by terciles of percentage of DAOH-AF.
Results: Among the 3387 patients included (median [IQR] age: 66.3 [57.9-70.9] years), 80.9% were male, 33.6% and 37.1% were Interfaces Patient Profile 2 and 3, respectively, and 61.1% received implants as destination therapy. Median (IQR) percent of DAOH-BF was 88.8% (82.7%-93.8%) and 84.6% (62.1-91.5%) for percent of DAOH-AF. While DAOH-BF was not associated with post-LVAD outcomes, patients in the low tercile of percentage of DAOH-AF had a longer index hospitalization stay (mean, 44 days; 95% CI, 16-77), were less likely to be discharged home (mean. -46.4 days; 95% CI, 44.2-49.1), and spent more time in a skilled nursing facility (mean, 27 days; 95% CI, 24-29), rehabilitation center (mean, 10 days; 95% CI, 8-12), or hospice (mean, 6 days; 95% CI, 4-8). Increasing percentage of DAOH-AF was associated with patient risk, AEs, and indices of HRQoL. Patients experiencing non-LVAD-related AEs had the lowest percentage of DAOH-AF.
Conclusions and relevance: Significant variability existed in the percentage of DAOH within a 1-year time horizon and was associated with the cumulative AEs burden. This patient-centered measure may assist clinicians in informing patients about expectations after durable LVAD implantation. Validation of percentage DAOH as a quality metric for LVAD therapy across centers should be explored.
Conflict of interest statement
Figures

Comment in
-
A Cautiously Optimistic Metric for Patients Undergoing Durable Left Ventricular Assist Device Implantation.JAMA Surg. 2023 Apr 1;158(4):e228138. doi: 10.1001/jamasurg.2022.8138. Epub 2023 Apr 12. JAMA Surg. 2023. PMID: 36811900 No abstract available.
References
-
- Yancy CW, Jessup M, Bozkurt B, et al. ; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines . 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62(16):e147-e239. doi: 10.1016/j.jacc.2013.05.019 - DOI - PubMed
-
- Aaronson KD, Slaughter MS, Miller LW, et al. ; HeartWare Ventricular Assist Device (HVAD) Bridge to Transplant ADVANCE Trial Investigators . Use of an intrapericardial, continuous-flow, centrifugal pump in patients awaiting heart transplantation. Circulation. 2012;125(25):3191-3200. doi: 10.1161/CIRCULATIONAHA.111.058412 - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

