Management of coronary artery disease in patients undergoing transcatheter aortic valve implantation. A clinical consensus statement from the European Association of Percutaneous Cardiovascular Interventions in collaboration with the ESC Working Group on Cardiovascular Surgery
- PMID: 36811935
- PMCID: PMC10174192
- DOI: 10.4244/EIJ-D-22-00958
Management of coronary artery disease in patients undergoing transcatheter aortic valve implantation. A clinical consensus statement from the European Association of Percutaneous Cardiovascular Interventions in collaboration with the ESC Working Group on Cardiovascular Surgery
Abstract
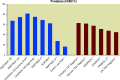
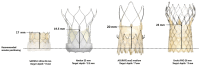
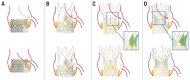
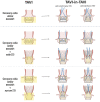
Significant coronary artery disease (CAD) is a frequent finding in patients with severe aortic stenosis undergoing transcatheter aortic valve implantation (TAVI), and the management of these two conditions becomes of particular importance with the extension of the procedure to younger and lower-risk patients. Yet, the preprocedural diagnostic evaluation and the indications for treatment of significant CAD in TAVI candidates remain a matter of debate. In this clinical consensus statement, a group of experts from the European Association of Percutaneous Cardiovascular Interventions (EAPCI) in collaboration with the European Society of Cardiology (ESC) Working Group on Cardiovascular Surgery aims to review the available evidence on the topic and proposes a rationale for the diagnostic evaluation and indications for percutaneous revascularisation of CAD in patients with severe aortic stenosis undergoing transcatheter treatment. Moreover, it also focuses on commissural alignment of transcatheter heart valves and coronary re-access after TAVI and redo-TAVI.
Conflict of interest statement
G. Tarantini reports lecture fees from Medtronic, Edwards Lifesciences, Abbott Vascular, and Boston Scientific. H. Eltchaninoff reports lecture fees from Edwards Lifesciences. D. Blackman reports consulting and lecture fees from Medtronic, Edwards Lifesciences, Abbott Vascular, and Boston Scientific. N. Bonaros reports lecture fees from Edwards Lifesciences and Medtronic. N. Karam reports consulting and lecture fees from Medtronic, Edwards Lifesciences, and Abbott Vascular. D. Mylotte reports consulting fees from Medtronic, Boston Scientific, and MicroPort. P. Carrilho-Ferreira reports lecture fees from Biotronik and Medtronic. N. Van Mieghem reports consulting fees from Biotronik, Boston Scientific, Abbott Vascular, Medtronic, PulseCath BV, and Abiomed; and research grants from Abbott Vascular, Edwards Lifesciences, Boston Scientific, Abiomed, Medtronic, PulseCath BV, Amgen, and Daiichi Sankyo. W-K. Kim reports lecture fees from Abbott, Boston Scientific, Edwards Lifesciences, Medtronic, and Meril Life Sciences. G. Tang is a physician proctor, physician advisory board member and consultant for Medtronic; physician advisory board member and consultant for Abbott Structural Heart; physician advisory board member for JenaValve; consultant for NeoChord; and has received speaker honoraria from Siemens Healthineers. O. De Backer received institutional research grants and consulting fees from Abbott, Boston Scientific, and Medtronic. L. Sondergaard received consultant fees and/or institutional research grants from Abbott, Boston Scientific, Medtronic, and SMT. The other authors have no conflicts of interest to declare.The Guest Editor reports lecture fees paid to his institution from Amgen, Bayer Healthcare, Biotronik, Boehringer Ingelheim, Boston Scientific, Daiichi Sankyo, Edwards Lifesciences, Ferrer, Pfizer, and Novartis; and consultancy fees paid to his institution from Boehringer.
Figures




References
-
- Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, Capodanno D, Conradi L, De Bonis, De Paulis, Delgado V, Freemantle N, Haugaa KH, Jeppsson A, Jüni P, Pierard L, Prendergast BD, Sádaba JR, Tribouilloy C, Wojakowski W. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. EuroIntervention. 2022;17:e1126–96. - PMC - PubMed
-
- Tarantini G, Nai Fovino, Gersh BJ. Transcatheter aortic valve implantation in lower-risk patients: what is the perspective? Eur Heart J. 2018;39:658–66. - PubMed
-
- Tarantini G, Lefèvre T, Terkelsen CJ, Frerker C, Ohlmann P, Mojoli M, Eltchaninoff H, Pinaud F, Redwood S, Windecker S. One-Year Outcomes of a European Transcatheter Aortic Valve Implantation Cohort According to Surgical Risk. Circ Cardiovasc Interv. 2019;12:e006724. - PubMed
-
- Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Brown DL, Block PC, Guyton RA, Pichard AD, Bavaria JE, Herrmann HC, Douglas PS, Petersen JL, Akin JJ, Anderson WN, Wang D, Pocock S PARTNER Trial Investigators. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597–607. - PubMed
-
- Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Williams M, Dewey T, Kapadia S, Babaliaros V, Thourani VH, Corso P, Pichard AD, Bavaria JE, Herrmann HC, Akin JJ, Anderson WN, Wang D, Pocock SJ PARTNER Trial Investigators. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364:2187–98. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

