Generating Fischer-Type Rh-Carbenes with Rh-Carbynoids
- PMID: 36812070
- PMCID: PMC9999426
- DOI: 10.1021/jacs.3c00012
Generating Fischer-Type Rh-Carbenes with Rh-Carbynoids
Abstract
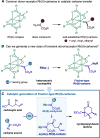
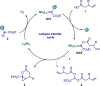
We describe the first catalytic generation of Fischer-type acyloxy Rh(II)-carbenes from carboxylic acids and Rh(II)-carbynoids. This novel class of transient donor/acceptor Rh(II)-carbenes evolved through a cyclopropanation process providing access to densely functionalized cyclopropyl-fused lactones with excellent diastereoselectivity. DFT calculations allowed the analysis of the properties of Rh(II)-carbynoids and acyloxy Rh(II)-carbenes as well as the characterization of the mechanism.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


Similar articles
-
Asymmetric multi-component trifunctionalization reactions with α-Halo Rh-carbenes.Nat Commun. 2025 Feb 7;16(1):1434. doi: 10.1038/s41467-025-56446-0. Nat Commun. 2025. PMID: 39920127 Free PMC article.
-
Catalytic Oxidative Ligand Transfer of Rh-Carbynoids and Alkyl Iodides.J Am Chem Soc. 2025 Sep 3;147(35):31444-31449. doi: 10.1021/jacs.5c09559. Epub 2025 Aug 21. J Am Chem Soc. 2025. PMID: 40841923 Free PMC article.
-
Enantioselective Synthesis of α-Aryl-β-Aminocyclopropane Carboxylic Acid Derivatives via Rh(II)-Catalyzed Cyclopropanation of Vinylsulfonamides with α-Aryldiazoesters.J Org Chem. 2022 Jan 21;87(2):1074-1085. doi: 10.1021/acs.joc.1c02386. Epub 2022 Jan 4. J Org Chem. 2022. PMID: 35057627
-
Catalytic Cleavage of C(sp2)-C(sp2) Bonds with Rh-Carbynoids.J Am Chem Soc. 2019 Oct 2;141(39):15509-15514. doi: 10.1021/jacs.9b08632. Epub 2019 Sep 19. J Am Chem Soc. 2019. PMID: 31514502
-
Computational Investigations of Heme Carbenes and Heme Carbene Transfer Reactions.Chemistry. 2019 Oct 17;25(58):13231-13247. doi: 10.1002/chem.201901984. Epub 2019 Aug 23. Chemistry. 2019. PMID: 31306529 Review.
Cited by
-
Ring expansion of indene by photoredox-enabled functionalized carbon-atom insertion.Nat Catal. 2024 Mar;7(3):242-251. doi: 10.1038/s41929-023-01089-x. Epub 2024 Jan 9. Nat Catal. 2024. PMID: 39512751 Free PMC article.
-
Diazomethyl-λ3-iodane meets aryne: dipolar cycloaddition and C-to-N iodane shift leading to indazolyl-λ3-iodanes.Chem Sci. 2025 Apr 2;16(18):8053-8059. doi: 10.1039/d5sc00266d. eCollection 2025 May 7. Chem Sci. 2025. PMID: 40206543 Free PMC article.
-
Metal-carbene-guided twofold cross-coupling of ethers with chromium catalysis.Nat Commun. 2024 Jul 31;15(1):6455. doi: 10.1038/s41467-024-50675-5. Nat Commun. 2024. PMID: 39085244 Free PMC article.
-
A Lead(II) Substituted Triplet Carbene.J Am Chem Soc. 2024 Oct 30;146(43):29630-29636. doi: 10.1021/jacs.4c10205. Epub 2024 Oct 18. J Am Chem Soc. 2024. PMID: 39423155 Free PMC article.
-
Manganese-catalyzed cyclopropanation of allylic alcohols with sulfones.Nat Commun. 2024 Aug 9;15(1):6798. doi: 10.1038/s41467-024-51188-x. Nat Commun. 2024. PMID: 39122745 Free PMC article.
References
-
-
For selected reviews:
- Doyle M. P.; Forbes D. C. Recent Advances in Asymmetric Catalytic Metal Carbene Transformations. Chem. Rev. 1998, 98, 911–936. 10.1021/cr940066a. - DOI - PubMed
- Davies H. M. L.; Beckwith R. E. J. Catalytic Enantioselective C-H Activation by Means of Metal-Carbenoid-Induced C-H Insertion. Chem. Rev. 2003, 103, 2861–2903. 10.1021/cr0200217. - DOI - PubMed
- Davies H. M. L.; Manning J. R. Catalytic C-H Functionalization by Metal Carbenoid and Nitrenoid Insertion. Nature 2008, 451, 417–424. 10.1038/nature06485. - DOI - PMC - PubMed
- Davies H. M. L.; Morton D. Guiding Principles for Site Selective and Stereoselective Intermolecular C–H Functionalization by Donor/Acceptor Rhodium Carbenes. Chem. Soc. Rev. 2011, 40, 1857–1869. 10.1039/c0cs00217h. - DOI - PubMed
- Davies H. M. L.; Liao K. Dirhodium Tetracarboxylates as Catalysts for Selective Intermolecular C–H Functionalization. Nat. Rev. Chem. 2019, 3, 347–360. 10.1038/s41570-019-0099-x. - DOI - PMC - PubMed
-
-
-
For characterization and isolation of donor/acceptor Rh(II)-carbenes:
- Kornecki K. P.; Briones J. F.; Boyarskikh V.; Fullilove F.; Autschbach J.; Schrote K. E.; Lancaster K. M.; Davies H. M. L.; Berry J. F. Direct Spectroscopic Characterization of a Transitory Dirhodium Donor-Acceptor Carbene Complex. Science 2013, 342, 351–354. 10.1126/science.1243200. - DOI - PubMed
- Werlé C.; Goddard R.; Fürstner A. The First Crystal Structure of a Reactive Dirhodium Carbene Complex and a Versatile Method for the Preparation of Gold Carbenes by Rhodium-to-Gold Transmetalation. Angew. Chem., Int. Ed. 2015, 54, 15452–15456. 10.1002/anie.201506902. - DOI - PMC - PubMed
- Werlé C.; Goddard R.; Philipps P.; Farès C.; Fürstner A. Structures of Reactive Donor/Acceptor and Donor/Donor Rhodium Carbenes in the Solid State and Their Implications for Catalysis. J. Am. Chem. Soc. 2016, 138, 3797–3805. 10.1021/jacs.5b13321. - DOI - PubMed
-
-
-
During the preparation of this manuscript, the group of Davies reported an elegant approach for the generation of aryloxy-subsituted Rh(II)-carbenes:
- Kubiak R. W.; Tracy W. F.; Alford J. S.; Davies H. M. L. Asymmetric Cyclopropanation with 4-Aryloxy-1-Sulfonyl-1,2,3-Triazoles: Expanding the Range of Rhodium-Stabilized Donor/Acceptor Carbenes to Systems with an Oxygen Donor Group. J. Org. Chem. 2022, 87, 13517–13528. 10.1021/acs.joc.2c00978. - DOI - PMC - PubMed
-
-
- Fischer E. O.; Maasböl A. On the Existence of a Tungsten Carbonyl Carbene Complex. Angew. Chem., Int. Ed. Engl. 1964, 3, 580–581. 10.1002/anie.196405801. - DOI
LinkOut - more resources
Full Text Sources

