Mitoxantrone targets both host and bacteria to overcome vancomycin resistance in Enterococcus faecalis
- PMID: 36812322
- PMCID: PMC9946351
- DOI: 10.1126/sciadv.add9280
Mitoxantrone targets both host and bacteria to overcome vancomycin resistance in Enterococcus faecalis
Abstract
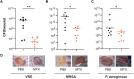
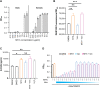
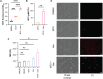
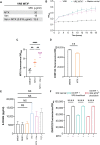
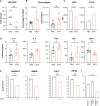
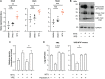
Antibiotic resistance critically limits treatment options for infection caused by opportunistic pathogens such as enterococci. Here, we investigate the antibiotic and immunological activity of the anticancer agent mitoxantrone (MTX) in vitro and in vivo against vancomycin-resistant Enterococcus faecalis (VRE). We show that, in vitro, MTX is a potent antibiotic against Gram-positive bacteria through induction of reactive oxygen species and DNA damage. MTX also synergizes with vancomycin against VRE, rendering the resistant strains more permeable to MTX. In a murine wound infection model, single-dose MTX treatment effectively reduces VRE numbers, with further reduction when combined with vancomycin. Multiple MTX treatments accelerate wound closure. MTX also promotes macrophage recruitment and proinflammatory cytokine induction at the wound site and augments intracellular bacterial killing in macrophages by up-regulating the expression of lysosomal enzymes. These results show that MTX represents a promising bacterium- and host-targeted therapeutic for overcoming vancomycin resistance.
Figures

References
-
- C. J. Murray, K. S. Ikuta, F. Sharara, L. Swetschinski, G. Robles Aguilar, A. Gray, C. Han, C. Bisignano, P. Rao, E. Wool, S. C. Johnson, A. J. Browne, M. G. Chipeta, F. Fell, S. Hackett, G. Haines-Woodhouse, B. H. Kashef Hamadani, E. A. P. Kumaran, B. McManigal, R. Agarwal, S. Akech, S. Albertson, J. Amuasi, J. Andrews, A. Aravkin, E. Ashley, F. Bailey, S. Baker, B. Basnyat, A. Bekker, R. Bender, A. Bethou, J. Bielicki, S. Boonkasidecha, J. Bukosia, C. Carvalheiro, C. Castañeda-Orjuela, V. Chansamouth, S. Chaurasia, S. Chiurchiù, F. Chowdhury, A. J. Cook, B. Cooper, T. R. Cressey, E. Criollo-Mora, M. Cunningham, S. Darboe, N. P. J. Day, M. de Luca, K. Dokova, A. Dramowski, S. J. Dunachie, T. Eckmanns, D. Eibach, A. Emami, N. Feasey, N. Fisher-Pearson, K. Forrest, D. Garrett, P. Gastmeier, A. Z. Giref, R. C. Greer, V. Gupta, S. Haller, A. Haselbeck, S. I. Hay, M. Holm, S. Hopkins, K. C. Iregbu, J. Jacobs, D. Jarovsky, F. Javanmardi, M. Khorana, N. Kissoon, E. Kobeissi, T. Kostyanev, F. Krapp, R. Krumkamp, A. Kumar, H. H. Kyu, C. Lim, D. Limmathurotsakul, M. J. Loftus, M. Lunn, J. Ma, N. Mturi, T. Munera-Huertas, P. Musicha, M. M. Mussi-Pinhata, T. Nakamura, R. Nanavati, S. Nangia, P. Newton, C. Ngoun, A. Novotney, D. Nwakanma, C. W. Obiero, A. Olivas-Martinez, P. Olliaro, E. Ooko, E. Ortiz-Brizuela, A. Y. Peleg, C. Perrone, N. Plakkal, A. Ponce-de-Leon, M. Raad, T. Ramdin, A. Riddell, T. Roberts, J. V. Robotham, A. Roca, K. E. Rudd, N. Russell, J. Schnall, J. A. G. Scott, M. Shivamallappa, J. Sifuentes-Osornio, N. Steenkeste, A. J. Stewardson, T. Stoeva, N. Tasak, A. Thaiprakong, G. Thwaites, C. Turner, P. Turner, H. R. van Doorn, S. Velaphi, A. Vongpradith, H. Vu, T. Walsh, S. Waner, T. Wangrangsimakul, T. Wozniak, P. Zheng, B. Sartorius, A. D. Lopez, A. Stergachis, C. Moore, C. Dolecek, M. Naghavi, Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 399, 629–655 (2022). - PMC - PubMed
-
- World Health Organization, New report calls for urgent action to avert antimicrobial resistance crisis; www.who.int/news/item/29-04-2019-new-report-calls-for-urgent-action-to-a...ink>.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

