Reduced insulin signaling in neurons induces sex-specific health benefits
- PMID: 36812323
- PMCID: PMC9946356
- DOI: 10.1126/sciadv.ade8137
Reduced insulin signaling in neurons induces sex-specific health benefits
Abstract
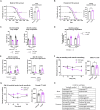
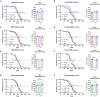
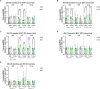
Reduced activity of insulin/insulin-like growth factor signaling (IIS) extends health and life span in mammals. Loss of the insulin receptor substrate 1 (Irs1) gene increases survival in mice and causes tissue-specific changes in gene expression. However, the tissues underlying IIS-mediated longevity are currently unknown. Here, we measured survival and health span in mice lacking IRS1 specifically in liver, muscle, fat, and brain. Tissue-specific loss of IRS1 did not increase survival, suggesting that lack of IRS1 in more than one tissue is required for life-span extension. Loss of IRS1 in liver, muscle, and fat did not improve health. In contrast, loss of neuronal IRS1 increased energy expenditure, locomotion, and insulin sensitivity, specifically in old males. Neuronal loss of IRS1 also caused male-specific mitochondrial dysfunction, activation of Atf4, and metabolic adaptations consistent with an activated integrated stress response at old age. Thus, we identified a male-specific brain signature of aging in response to reduced IIS associated with improved health at old age.
Figures


Similar articles
-
Evidence for lifespan extension and delayed age-related biomarkers in insulin receptor substrate 1 null mice.FASEB J. 2008 Mar;22(3):807-18. doi: 10.1096/fj.07-9261com. Epub 2007 Oct 10. FASEB J. 2008. PMID: 17928362
-
Reduced insulin/insulin-like growth factor signaling decreases translation in Drosophila and mice.Sci Rep. 2016 Jul 25;6:30290. doi: 10.1038/srep30290. Sci Rep. 2016. PMID: 27452396 Free PMC article.
-
Longevity in response to lowered insulin signaling requires glycine N-methyltransferase-dependent spermidine production.Aging Cell. 2020 Jan;19(1):e13043. doi: 10.1111/acel.13043. Epub 2019 Nov 13. Aging Cell. 2020. PMID: 31721422 Free PMC article.
-
Insulin and aging.Cell Cycle. 2008 Nov 1;7(21):3338-43. doi: 10.4161/cc.7.21.7012. Epub 2008 Nov 15. Cell Cycle. 2008. PMID: 18948730 Review.
-
Sex-Specific Gene Expression and Life Span Regulation.Trends Endocrinol Metab. 2017 Oct;28(10):735-747. doi: 10.1016/j.tem.2017.07.002. Epub 2017 Aug 2. Trends Endocrinol Metab. 2017. PMID: 28780002 Free PMC article. Review.
Cited by
-
Changes of Signaling Pathways in Hypothalamic Neurons with Aging.Curr Issues Mol Biol. 2023 Oct 12;45(10):8289-8308. doi: 10.3390/cimb45100523. Curr Issues Mol Biol. 2023. PMID: 37886966 Free PMC article. Review.
-
Sex-specific effects of Cre expression in Syn1Cre mice.Sci Rep. 2023 Jun 20;13(1):10037. doi: 10.1038/s41598-023-37029-9. Sci Rep. 2023. PMID: 37340054 Free PMC article.
-
The geroprotectors trametinib and rapamycin combine additively to extend mouse healthspan and lifespan.Nat Aging. 2025 Jul;5(7):1249-1265. doi: 10.1038/s43587-025-00876-4. Epub 2025 May 28. Nat Aging. 2025. PMID: 40437307 Free PMC article.
-
Ribosomal S6 kinase 1 regulates inflammaging via the senescence secretome.Nat Aging. 2024 Nov;4(11):1544-1561. doi: 10.1038/s43587-024-00695-z. Epub 2024 Aug 29. Nat Aging. 2024. PMID: 39210150 Free PMC article.
-
The Regulation of Cellular Senescence in Cancer.Biomolecules. 2025 Mar 20;15(3):448. doi: 10.3390/biom15030448. Biomolecules. 2025. PMID: 40149983 Free PMC article. Review.
References
-
- L. Partridge, J. Deelen, P. E. Slagboom, Facing up to the global challenges of ageing. Nature 561, 45–56 (2018). - PubMed
-
- C. J. Kenyon, The genetics of ageing. Nature 464, 504–512 (2010). - PubMed
-
- M. D. W. Piper, L. Partridge, Drosophila as a model for ageing. Biochim. Biophys. Acta Mol. Basis Dis. 1864, 2707–2717 (2018). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

