Combination of Multiple Low-Risk Lifestyle Behaviors and Incident Type 2 Diabetes: A Systematic Review and Dose-Response Meta-analysis of Prospective Cohort Studies
- PMID: 36812419
- PMCID: PMC10020027
- DOI: 10.2337/dc22-1024
Combination of Multiple Low-Risk Lifestyle Behaviors and Incident Type 2 Diabetes: A Systematic Review and Dose-Response Meta-analysis of Prospective Cohort Studies
Abstract
Objective: Combined low-risk lifestyle behaviors (LRLBs) have been associated with a reduction in type 2 diabetes risk. This relationship has not been systematically quantified.
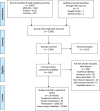
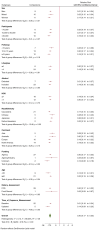
Research design and methods: A systematic review and meta-analysis was conducted to assess the association of combined LRLBs with type 2 diabetes. Databases were searched up to September 2022. Prospective cohort studies reporting the association between a minimum of three combined LRLBs (including healthy diet) with incident type 2 diabetes were included. Independent reviewers extracted data and assessed study quality. Risk estimates of extreme comparisons were pooled using a random-effects model. Global dose-response meta-analysis (DRM) for maximum adherence was estimated using a one-stage linear mixed model. The certainty of the evidence was assessed using GRADE (Grading of Recommendations, Assessment, Development and Evaluations).
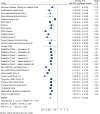
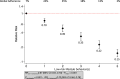
Results: Thirty cohort comparisons (n = 1,693,753) involving 75,669 incident type 2 diabetes cases were included. LRLBs, with author-defined ranges, were healthy body weight, healthy diet, regular exercise, smoking abstinence or cessation, and light alcohol consumption. LRLBs were associated with 80% lower risk of type 2 diabetes (relative risk [RR] 0.20; 95% CI 0.17-0.23), comparing the highest with lowest adherence. Global DRM for maximum adherence to all five LRLBs reached 85% protection (RR 0.15; 95% CI 0.12-0.18). The overall certainty of the evidence was graded as high.
Conclusions: There is a very good indication that a combination of LRLBs that includes maintaining a healthy bodyweight, healthy diet, regular exercise, smoking abstinence or cessation, and light alcohol consumption is associated with a lower risk of incident type 2 diabetes.
© 2023 by the American Diabetes Association.
Conflict of interest statement
Figures

Similar articles
-
Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: a network meta-analysis.Cochrane Database Syst Rev. 2020 Oct 19;10(10):CD012859. doi: 10.1002/14651858.CD012859.pub2. Cochrane Database Syst Rev. 2020. PMID: 33075160 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
Selenium for preventing cancer.Cochrane Database Syst Rev. 2018 Jan 29;1(1):CD005195. doi: 10.1002/14651858.CD005195.pub4. Cochrane Database Syst Rev. 2018. PMID: 29376219 Free PMC article.
-
Lifestyle interventions for the treatment of women with gestational diabetes.Cochrane Database Syst Rev. 2017 May 4;5(5):CD011970. doi: 10.1002/14651858.CD011970.pub2. Cochrane Database Syst Rev. 2017. PMID: 28472859 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
Cited by
-
Exploring the Link between Lifestyle, Inflammation, and Insulin Resistance through an Improved Healthy Living Index.Nutrients. 2024 Jan 29;16(3):388. doi: 10.3390/nu16030388. Nutrients. 2024. PMID: 38337673 Free PMC article.
-
Analysis of ancestry-specific polygenic risk score and diet composition in type 2 diabetes.PLoS One. 2023 May 23;18(5):e0285827. doi: 10.1371/journal.pone.0285827. eCollection 2023. PLoS One. 2023. PMID: 37220136 Free PMC article.
-
Clinical value of serum miR-214-3p expression in the diagnosis of type 2 diabetes mellitus and prediction of its chronic complications.BMC Endocr Disord. 2025 Apr 14;25(1):98. doi: 10.1186/s12902-025-01916-1. BMC Endocr Disord. 2025. PMID: 40229736 Free PMC article.
-
An evidence-based assessment of the nutritional recommendations for the prevention of diabetes mellitus.Hormones (Athens). 2025 Mar;24(1):59-70. doi: 10.1007/s42000-024-00604-4. Epub 2024 Sep 17. Hormones (Athens). 2025. PMID: 39287760
-
Oxidative Balance Score and New-Onset Type 2 Diabetes Mellitus in Korean Adults without Non-Alcoholic Fatty Liver Disease: Korean Genome and Epidemiology Study-Health Examinees (KoGES-HEXA) Cohort.Antioxidants (Basel). 2024 Jan 16;13(1):107. doi: 10.3390/antiox13010107. Antioxidants (Basel). 2024. PMID: 38247531 Free PMC article.
References
-
- Saeedi P, Petersohn I, Salpea P, et al. .; IDF Diabetes Atlas Committee . Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract 2019;157:107843. - PubMed
-
- Schienkiewitz A, Schulze MB, Hoffmann K, Kroke A, Boeing H. Body mass index history and risk of type 2 diabetes: results from the European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Am J Clin Nutr 2006;84:427–433 - PubMed
-
- Jannasch F, Kröger J, Schulze MB. Dietary patterns and type 2 diabetes: a systematic literature review and meta-analysis of prospective studies. J Nutr 2017;147:1174–1182 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

