The potential of digital molecular diagnostics for infectious diseases in sub-Saharan Africa
- PMID: 36812544
- PMCID: PMC9931288
- DOI: 10.1371/journal.pdig.0000064
The potential of digital molecular diagnostics for infectious diseases in sub-Saharan Africa
Erratum in
-
Correction: The potential of digital molecular diagnostics for infectious diseases in sub-Saharan Africa.PLOS Digit Health. 2022 Aug 30;1(8):e0000105. doi: 10.1371/journal.pdig.0000105. eCollection 2022 Aug. PLOS Digit Health. 2022. PMID: 36812640 Free PMC article.
Abstract
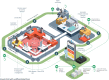
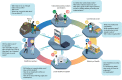
There is a large gap between diagnostic needs and diagnostic access across much of sub-Saharan Africa (SSA), particularly for infectious diseases that inflict a substantial burden of morbidity and mortality. Accurate diagnostics are essential for the correct treatment of individuals and provide vital information underpinning disease surveillance, prevention, and control strategies. Digital molecular diagnostics combine the high sensitivity and specificity of molecular detection with point-of-care format and mobile connectivity. Recent developments in these technologies create an opportunity for a radical transformation of the diagnostic ecosystem. Rather than trying to emulate diagnostic laboratory models in resource-rich settings, African countries have the potential to pioneer new models of healthcare designed around digital diagnostics. This article describes the need for new diagnostic approaches, highlights advances in digital molecular diagnostic technology, and outlines their potential for tackling infectious diseases in SSA. It then addresses the steps that will be necessary for the development and implementation of digital molecular diagnostics. Although the focus is on infectious diseases in SSA, many of the principles apply to other resource-limited settings and to noncommunicable diseases.
Copyright: © 2022 The Digital Diagnostics for Africa Network. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
I have read the journal’s policy and the authors of this manuscript have the following competing interests: P.G., J.R-M., and N.M, have a start-up company, ProtonDx Ltd, producing a digital diagnostic for detection of SARS-CoV-2. P.G., J.R.-M. and J.B. are named authors on a patent that covers methodology for detection of single nucleotide polymorphisms that can be used in a digital diagnostic for malaria. All other authors declare no conflicts of interest.
Figures



References
-
- Azevedo MJ. The State of Health System(s) in Africa: Challenges and Opportunities. In: Historical Perspectives on the State of Health and Health Systems in Africa, Volume II. Cham: Palgrave Macmillan; 2017. pp. 1–73.
-
- World Health Organisation. Tracking Universal Health Coverage: 2017 Global Monitoring Report: executive summary. 2017. [cited 2020 Nov 10]. Available from: https://apps.who.int/iris/bitstream/handle/10665/259817/9789241513555-en....
-
- Plianbangchang S. Universal Health Coverage (UHC). J Heal Res. 2018. Aug 3;32(4):322–4.
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
