Artificial intelligence applications used in the clinical response to COVID-19: A scoping review
- PMID: 36812557
- PMCID: PMC9931281
- DOI: 10.1371/journal.pdig.0000132
Artificial intelligence applications used in the clinical response to COVID-19: A scoping review
Abstract
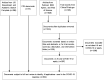
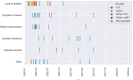
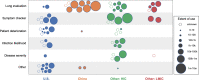
Research into using artificial intelligence (AI) in health care is growing and several observers predicted that AI would play a key role in the clinical response to the COVID-19. Many AI models have been proposed though previous reviews have identified only a few applications used in clinical practice. In this study, we aim to (1) identify and characterize AI applications used in the clinical response to COVID-19; (2) examine the timing, location, and extent of their use; (3) examine how they relate to pre-pandemic applications and the U.S. regulatory approval process; and (4) characterize the evidence that is available to support their use. We searched academic and grey literature sources to identify 66 AI applications that performed a wide range of diagnostic, prognostic, and triage functions in the clinical response to COVID-19. Many were deployed early in the pandemic and most were used in the U.S., other high-income countries, or China. While some applications were used to care for hundreds of thousands of patients, others were used to an unknown or limited extent. We found studies supporting the use of 39 applications, though few of these were independent evaluations and we found no clinical trials evaluating any application's impact on patient health. Due to limited evidence, it is impossible to determine the extent to which the clinical use of AI in the pandemic response has benefited patients overall. Further research is needed, particularly independent evaluations on AI application performance and health impacts in real-world care settings.
Copyright: © 2022 Mann et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Beyond the black stump: rapid reviews of health research issues affecting regional, rural and remote Australia.Med J Aust. 2020 Dec;213 Suppl 11:S3-S32.e1. doi: 10.5694/mja2.50881. Med J Aust. 2020. PMID: 33314144
-
The Impact of Artificial Intelligence on Health Equity in Oncology: Scoping Review.J Med Internet Res. 2022 Nov 1;24(11):e39748. doi: 10.2196/39748. J Med Internet Res. 2022. PMID: 36005841 Free PMC article.
-
Artificial intelligence for strengthening healthcare systems in low- and middle-income countries: a systematic scoping review.NPJ Digit Med. 2022 Oct 28;5(1):162. doi: 10.1038/s41746-022-00700-y. NPJ Digit Med. 2022. PMID: 36307479 Free PMC article.
-
Artificial Intelligence (AI) for Screening Mammography, From the AJR Special Series on AI Applications.AJR Am J Roentgenol. 2022 Sep;219(3):369-380. doi: 10.2214/AJR.21.27071. Epub 2022 Jan 12. AJR Am J Roentgenol. 2022. PMID: 35018795 Review.
-
Artificial Intelligence Applications in Health Care Practice: Scoping Review.J Med Internet Res. 2022 Oct 5;24(10):e40238. doi: 10.2196/40238. J Med Internet Res. 2022. PMID: 36197712 Free PMC article.
Cited by
-
Good practices for clinical data warehouse implementation: A case study in France.PLOS Digit Health. 2023 Jul 6;2(7):e0000298. doi: 10.1371/journal.pdig.0000298. eCollection 2023 Jul. PLOS Digit Health. 2023. PMID: 37410797 Free PMC article. Review.
-
What proportion of clinical prediction models make it to clinical practice? Protocol for a two-track follow-up study of prediction model development publications.BMJ Open. 2023 May 17;13(5):e073174. doi: 10.1136/bmjopen-2023-073174. BMJ Open. 2023. PMID: 37197813 Free PMC article.
-
Extracting relevant predictive variables for COVID-19 severity prognosis: An exhaustive comparison of feature selection techniques.PLoS One. 2023 Apr 13;18(4):e0284150. doi: 10.1371/journal.pone.0284150. eCollection 2023. PLoS One. 2023. PMID: 37053151 Free PMC article.
-
Mixed methods assessment of the influence of demographics on medical advice of ChatGPT.J Am Med Inform Assoc. 2024 Sep 1;31(9):2002-2009. doi: 10.1093/jamia/ocae086. J Am Med Inform Assoc. 2024. PMID: 38679900 Free PMC article.
-
Emerging healthcare interventions: Patient-Centered Outcomes Research Institute's programmatic initiative.Int J Technol Assess Health Care. 2023 Jun 20;39(1):e36. doi: 10.1017/S0266462323000284. Int J Technol Assess Health Care. 2023. PMID: 37336780 Free PMC article. Review.
References
-
- U.S. Food and Drug Administration. Artificial Intelligence and Machine Learning (AI/ML)-Enabled Medical Devices 2021 [updated 2021 Sep 22; cited 2022 June 26]. Available from: https://www.fda.gov/medical-devices/software-medical-device-samd/artific....
-
- Bestsennyy O, Gilbert G, Harris A, Rost J. Telehealth: A quarter-trillion-dollar post-COVID-19 reality? McKinsey. 2021. July 9 [cited 2022 May 31]. Available from: https://www.mckinsey.com/industries/healthcare-systems-and-services/our-....
Publication types
LinkOut - more resources
Full Text Sources
