A proposed de-identification framework for a cohort of children presenting at a health facility in Uganda
- PMID: 36812586
- PMCID: PMC9931294
- DOI: 10.1371/journal.pdig.0000027
A proposed de-identification framework for a cohort of children presenting at a health facility in Uganda
Abstract
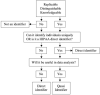
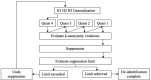
Data sharing has enormous potential to accelerate and improve the accuracy of research, strengthen collaborations, and restore trust in the clinical research enterprise. Nevertheless, there remains reluctancy to openly share raw data sets, in part due to concerns regarding research participant confidentiality and privacy. Statistical data de-identification is an approach that can be used to preserve privacy and facilitate open data sharing. We have proposed a standardized framework for the de-identification of data generated from cohort studies in children in a low-and-middle income country. We applied a standardized de-identification framework to a data sets comprised of 241 health related variables collected from a cohort of 1750 children with acute infections from Jinja Regional Referral Hospital in Eastern Uganda. Variables were labeled as direct and quasi-identifiers based on conditions of replicability, distinguishability, and knowability with consensus from two independent evaluators. Direct identifiers were removed from the data sets, while a statistical risk-based de-identification approach using the k-anonymity model was applied to quasi-identifiers. Qualitative assessment of the level of privacy invasion associated with data set disclosure was used to determine an acceptable re-identification risk threshold, and corresponding k-anonymity requirement. A de-identification model using generalization, followed by suppression was applied using a logical stepwise approach to achieve k-anonymity. The utility of the de-identified data was demonstrated using a typical clinical regression example. The de-identified data sets was published on the Pediatric Sepsis Data CoLaboratory Dataverse which provides moderated data access. Researchers are faced with many challenges when providing access to clinical data. We provide a standardized de-identification framework that can be adapted and refined based on specific context and risks. This process will be combined with moderated access to foster coordination and collaboration in the clinical research community.
Copyright: © 2022 Mawji et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
JMA serves as a section editor for PLOS Digital Health. The peer-review process was guided by an independent editor, and the authors have no other competing interests to declare.
Figures

References
-
- Government of Canada [Internet]. Open data 101; 2020 [cited 2022 Jan 27]. Available from https://open.canada.ca/en/open-data-principles.
-
- Gates Open Research [Internet]. Data guidelines; 2022 [cited 2022 Jan 27]. Available from https://gatesopenresearch.org/for-authors/data-guidelines.
-
- PLOS [Internet]. Open data; 2022 [cited 2022 Jan 27]. Available from https://plos.org/open-science/open-data/.
LinkOut - more resources
Full Text Sources
