Breakthroughs in Hepatocellular Carcinoma Therapies
- PMID: 36813012
- PMCID: PMC10293061
- DOI: 10.1016/j.cgh.2023.01.039
Breakthroughs in Hepatocellular Carcinoma Therapies
Abstract
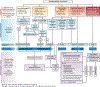
Several breakthroughs in hepatocellular carcinoma (HCC) therapy across tumor stages provide hope to improve its dismal prognosis. Although surgical and local ablative therapies have few significant changes in technique, an improved understanding of tumor biology has facilitated increase numbers of patients who are now eligible to undergo curative-intent procedures. Most notably, acceptable post-transplant outcomes can be achieved in well selected patients whose tumors are downstaged into Milan Criteria. Adjuvant therapy in patients at high risk of recurrence also significantly improves recurrence-free survival after resection or ablation. For patients with liver-localized disease who are not eligible for curative-intent procedures, transarterial chemoembolization (TACE) was historically the treatment modality of choice, regardless of tumor burden; however, there is now increased recognition of patients who are "TACE unsuitable" and may be better treated with systemic therapy. The greatest evolution in HCC treatment options has occurred with systemic therapy, where several new agents are now available in the first- and second-line setting, including immune checkpoint inhibitor combinations. Objective responses are observed in approximately 30% of patients and median survival is approaching 2 years. The availability of immune checkpoint inhibitors has renewed interest in combination therapies for earlier tumor stages, with several phase III trials ongoing. Considering increasing complexities of HCC care, requiring decisions between therapies delivered by different providers, multidisciplinary care is critical and is associated with improved clinical outcomes. In this review, we detail major breakthroughs in HCC therapy, how these breakthroughs can be applied in clinical practice, and remaining areas in need of further research.
Keywords: ablation; hepatocellular carcinoma; liver transplantation; molecular targeted therapy; resection; transarterial chemoembolization.
Copyright © 2023 AGA Institute. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures


References
-
- Vogel A, Meyer T, Sapisochin G, et al. Hepatocellular carcinoma. Lancet 2022;400:1345–1362. - PubMed
-
- Citterio D, Facciorusso A, Sposito C, et al. Hierarchic Interaction of Factors Associated With Liver Decompensation After Resection for Hepatocellular Carcinoma. JAMA Surg 2016;151:846–853. - PubMed
-
- Tsilimigras DI, Bagante F, Moris D, et al. Defining the chance of cure after resection for hepatocellular carcinoma within and beyond the Barcelona Clinic Liver Cancer guidelines: A multi-institutional analysis of 1,010 patients. Surgery 2019;166:967–974. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

