Neural correlates of impulsivity in bipolar disorder: A systematic review and clinical implications
- PMID: 36813146
- PMCID: PMC11073484
- DOI: 10.1016/j.neubiorev.2023.105109
Neural correlates of impulsivity in bipolar disorder: A systematic review and clinical implications
Abstract
Impulsivity is a common feature of bipolar disorder (BD) with ramifications for functional impairment and premature mortality. This PRISMA-guided systematic review aims to integrate findings on the neurocircuitry associated with impulsivity in BD. We searched for functional neuroimaging studies that examined rapid-response impulsivity and choice impulsivity using the Go/No-Go Task, Stop-Signal Task, and Delay Discounting Task. Findings from 33 studies were synthesized with an emphasis on the effect of mood state of the sample and affective salience of the task. Results suggest trait-like brain activation abnormalities in regions implicated in impulsivity that persist across mood states. During rapid-response inhibition, BD exhibit under-activation of key frontal, insular, parietal, cingulate, and thalamic regions, but over-activation of these regions when the task involves emotional stimuli. Delay discounting tasks with functional neuroimaging in BD are lacking, but hyperactivity of orbitofrontal and striatal regions associated with reward hypersensitivity may be related to difficulty delaying gratification. We propose a working model of neurocircuitry dysfunction underlying behavioral impulsivity in BD. Clinical implications and future directions are discussed.
Keywords: Choice impulsivity; Delay discounting; Functional neuroimaging; Go/no-go; Inhibition; Neurocircuitry; Rapid-response impulsivity; Stop-signal task.
Published by Elsevier Ltd.
Conflict of interest statement
Declarations of interest
None.
Figures
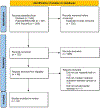
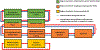
References
-
- American Psychiatric Association, 2013. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, ed. American Psychiatric Association. 10.1176/appi.books.9780890425596. - DOI

