Inhaled ciclesonide in adults hospitalised with COVID-19: a randomised controlled open-label trial (HALT COVID-19)
- PMID: 36813503
- PMCID: PMC9950582
- DOI: 10.1136/bmjopen-2022-064374
Inhaled ciclesonide in adults hospitalised with COVID-19: a randomised controlled open-label trial (HALT COVID-19)
Abstract
Objective: To assess the efficacy of inhaled ciclesonide in reducing the duration of oxygen therapy (an indicator of time to clinical improvement) among adults hospitalised with COVID-19.
Design: Multicentre, randomised, controlled, open-label trial.
Setting: 9 hospitals (3 academic hospitals and 6 non-academic hospitals) in Sweden between 1 June 2020 and 17 May 2021.
Participants: Adults hospitalised with COVID-19 and receiving oxygen therapy.
Intervention: Inhaled ciclesonide 320 µg two times a day for 14 days versus standard care.
Main outcome measures: Primary outcome was duration of oxygen therapy, an indicator of time to clinical improvement. Key secondary outcome was a composite of invasive mechanical ventilation/death.
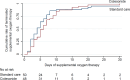
Results: Data from 98 participants were analysed (48 receiving ciclesonide and 50 receiving standard care; median (IQR) age, 59.5 (49-67) years; 67 (68%) men). Median (IQR) duration of oxygen therapy was 5.5 (3-9) days in the ciclesonide group and 4 (2-7) days in the standard care group (HR for termination of oxygen therapy 0.73 (95% CI 0.47 to 1.11), with the upper 95% CI being compatible with a 10% relative reduction in oxygen therapy duration, corresponding to a <1 day absolute reduction in a post-hoc calculation). Three participants in each group died/received invasive mechanical ventilation (HR 0.90 (95% CI 0.15 to 5.32)). The trial was discontinued early due to slow enrolment.
Conclusions: In patients hospitalised with COVID-19 receiving oxygen therapy, this trial ruled out, with 0.95 confidence, a treatment effect of ciclesonide corresponding to more than a 1 day reduction in duration of oxygen therapy. Ciclesonide is unlikely to improve this outcome meaningfully.
Trial registration number: NCT04381364.
Keywords: COVID-19; clinical trials; respiratory infections.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: This study received non-financial support from COVIS Pharma (study drug donation). The authors have no conflict of interest to disclose.
Figures
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical