Effectiveness of analgesic ear drops as add-on treatment to oral analgesics in children with acute otitis media: study protocol of the OPTIMA pragmatic randomised controlled trial
- PMID: 36813504
- PMCID: PMC9950909
- DOI: 10.1136/bmjopen-2022-062071
Effectiveness of analgesic ear drops as add-on treatment to oral analgesics in children with acute otitis media: study protocol of the OPTIMA pragmatic randomised controlled trial
Abstract
Introduction: Ear pain is the most prominent symptom of childhood acute otitis media (AOM). To control the pain and reduce reliance on antibiotics, evidence of effectiveness for alternative interventions is urgently needed. This trial aims to investigate whether analgesic ear drops added to usual care provide superior ear pain relief over usual care alone in children presenting to primary care with AOM.
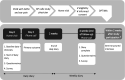
Methods and analysis: This is a pragmatic, two-arm, individually randomised, open, superiority trial with cost-effectiveness analysis and nested mixed-methods process evaluation in general practices in the Netherlands. We aim to recruit 300 children aged 1-6 years with a general practitioner (GP) diagnosis of AOM and ear pain. Children will be randomly allocated (ratio 1:1) to either (1) lidocaine hydrochloride 5 mg/g ear drops (Otalgan) one to two drops up to six times daily for a maximum of 7 days in addition to usual care (oral analgesics, with/without antibiotics); or (2) usual care. Parents will complete a symptom diary for 4 weeks as well as generic and disease-specific quality of life questionnaires at baseline and 4 weeks. The primary outcome is the parent-reported ear pain score (0-10) over the first 3 days. Secondary outcomes include proportion of children consuming antibiotics, oral analgesic use and overall symptom burden in the first 7 days; number of days with ear pain, number of GP reconsultations and subsequent antibiotic prescribing, adverse events, complications of AOM and cost-effectiveness during 4-week follow-up; generic and disease-specific quality of life at 4 weeks; parents' and GPs' views and experiences with treatment acceptability, usability and satisfaction.
Ethics and dissemination: The Medical Research Ethics Committee Utrecht, the Netherlands, has approved the protocol (21-447/G-D). All parents/guardians of participants will provide written informed consent. Study results will be submitted for publication in peer-reviewed medical journals and presented at relevant (inter)national scientific meetings.
Trial registration: The Netherlands Trial Register: NL9500; date of registration: 28 May 2021. At the time of publication of the study protocol paper, we were unable to make any amendments to the trial registration record in the Netherlands Trial Register. The addition of a data sharing plan was required to adhere to the International Committee of Medical Journal Editors guidelines. The trial was therefore reregistered in ClinicalTrials.gov (NCT05651633; date of registration: 15 December 2022). This second registration is for modification purposes only and the Netherlands Trial Register record (NL9500) should be regarded as the primary trial registration.
Keywords: Epidemiology; PAIN MANAGEMENT; PRIMARY CARE; Paediatric infectious disease & immunisation; Paediatric otolaryngology; QUALITATIVE RESEARCH.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
Similar articles
-
Anaesthetic-analgesic ear drops to reduce antibiotic consumption in children with acute otitis media: the CEDAR RCT.Health Technol Assess. 2019 Jul;23(34):1-48. doi: 10.3310/hta23340. Health Technol Assess. 2019. PMID: 31304912 Free PMC article. Clinical Trial.
-
Topical or oral antibiotics for children with acute otitis media presenting with ear discharge: study protocol of a randomised controlled non-inferiority trial.BMJ Open. 2021 Dec 16;11(12):e052128. doi: 10.1136/bmjopen-2021-052128. BMJ Open. 2021. PMID: 34916313 Free PMC article.
-
Immediate oral versus immediate topical versus delayed oral antibiotics for children with acute otitis media with discharge: the REST three-arm non-inferiority electronic platform-supported RCT.Health Technol Assess. 2021 Nov;25(67):1-76. doi: 10.3310/hta25670. Health Technol Assess. 2021. PMID: 34816795 Clinical Trial.
-
Impact of acute otitis media clinical practice guidelines on antibiotic and analgesic prescriptions: a systematic review.Arch Dis Child. 2018 Jun;103(6):597-602. doi: 10.1136/archdischild-2017-314103. Epub 2018 Mar 3. Arch Dis Child. 2018. PMID: 29502073 Free PMC article.
-
Pain Relief by Analgesic Eardrops: Paradigm Shift in the Treatment of Acute Otitis Media?Drug Res (Stuttg). 2021 Sep;71(7):363-371. doi: 10.1055/a-1494-3087. Epub 2021 Jun 7. Drug Res (Stuttg). 2021. PMID: 34098586 Review.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical