Microbiota-derived 3-IAA influences chemotherapy efficacy in pancreatic cancer
- PMID: 36813961
- PMCID: PMC9977685
- DOI: 10.1038/s41586-023-05728-y
Microbiota-derived 3-IAA influences chemotherapy efficacy in pancreatic cancer
Erratum in
-
Author Correction: Microbiota-derived 3-IAA influences chemotherapy efficacy in pancreatic cancer.Nature. 2025 May;641(8065):E12-E13. doi: 10.1038/s41586-025-08979-z. Nature. 2025. PMID: 40360841 Free PMC article. No abstract available.
Abstract
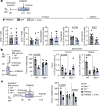
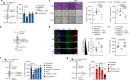
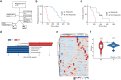
Pancreatic ductal adenocarcinoma (PDAC) is expected to be the second most deadly cancer by 2040, owing to the high incidence of metastatic disease and limited responses to treatment1,2. Less than half of all patients respond to the primary treatment for PDAC, chemotherapy3,4, and genetic alterations alone cannot explain this5. Diet is an environmental factor that can influence the response to therapies, but its role in PDAC is unclear. Here, using shotgun metagenomic sequencing and metabolomic screening, we show that the microbiota-derived tryptophan metabolite indole-3-acetic acid (3-IAA) is enriched in patients who respond to treatment. Faecal microbiota transplantation, short-term dietary manipulation of tryptophan and oral 3-IAA administration increase the efficacy of chemotherapy in humanized gnotobiotic mouse models of PDAC. Using a combination of loss- and gain-of-function experiments, we show that the efficacy of 3-IAA and chemotherapy is licensed by neutrophil-derived myeloperoxidase. Myeloperoxidase oxidizes 3-IAA, which in combination with chemotherapy induces a downregulation of the reactive oxygen species (ROS)-degrading enzymes glutathione peroxidase 3 and glutathione peroxidase 7. All of this results in the accumulation of ROS and the downregulation of autophagy in cancer cells, which compromises their metabolic fitness and, ultimately, their proliferation. In humans, we observed a significant correlation between the levels of 3-IAA and the efficacy of therapy in two independent PDAC cohorts. In summary, we identify a microbiota-derived metabolite that has clinical implications in the treatment of PDAC, and provide a motivation for considering nutritional interventions during the treatment of patients with cancer.
© 2023. The Author(s).
Conflict of interest statement
D.Z. received travel support from AstraZeneca and AMGEN, honoraria from AstraZeneca and Roche. S.B. reports honoraria from AstraZeneca, BMS, Celgene, Incyte, Janssen Cilag, MSD and Servier for scientific presentations and paid consulting, as well as research support from Celgene. J.T.S. receives honoraria as consultant or for continuing medical education presentations from AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Immunocore, MSD Sharp Dohme, Novartis, Roche/Genentech, and Servier. His institution receives research funding from Abalos Therapeutics, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Eisbach Bio, and Roche/Genentech; he holds ownership and serves on the Board of Directors of Pharma15. C.B. reports personal fees from Sanofi Aventis, Merck KgA, Bristol-Myers Squibb, Merck Sharp & Dohme, Lilly Imclone, Bayer Healthcare, GSO Contract Research, AOK Rheinland-Hamburg and Novartis. M. Sinn received honoraria from AstraZeneca, Amgen, BMS, MSD, Incyte, Pierre Fabre, Pfizer Servier and Sanofi and support for clinical research (institutional) from Amgen, AstraZeneca, Bayer, BMS, Incyte, MSD, Pierre Fabre, Roche and Servier. N.G. reports financial support from Roche. All of these are outside the submitted work. A.C.K. has financial interests in Vescor Therapeutics, OncoRev and is an inventor on patents pertaining to KRAS-regulated metabolic pathways and redox control pathways in pancreatic cancer, targeting GOT1 as a therapeutic approach, targeting alanine transport, and the autophagic control of iron metabolism. A.C.K. is on the scientific advisory board of Rafael/Cornerstone Pharmaceuticals and has been a consultant for Deciphera and Abbvie. The remaining authors declare no competing interests.
Figures










Comment in
-
A gut reaction can tune tumour fate during chemotherapy.Nature. 2023 Mar;615(7950):36-37. doi: 10.1038/d41586-023-00476-5. Nature. 2023. PMID: 36814015 No abstract available.
-
Microbiota-derived metabolites in PDAC.Nat Rev Gastroenterol Hepatol. 2023 May;20(5):270. doi: 10.1038/s41575-023-00767-2. Nat Rev Gastroenterol Hepatol. 2023. PMID: 36914826 No abstract available.
-
Tryptophan metabolite improves PDAC response rates and survival.Nat Rev Cancer. 2023 May;23(5):272. doi: 10.1038/s41568-023-00566-6. Nat Rev Cancer. 2023. PMID: 36973408 No abstract available.
-
A gut response: Modulating chemotherapy efficacy with microbial metabolites.Immunity. 2023 Apr 11;56(4):750-752. doi: 10.1016/j.immuni.2023.03.011. Immunity. 2023. PMID: 37044066
-
From bugs to drugs: Bacterial 3-IAA enhances efficacy of chemotherapy in pancreatic cancer.Cell Rep Med. 2023 May 16;4(5):101039. doi: 10.1016/j.xcrm.2023.101039. Cell Rep Med. 2023. PMID: 37196631 Free PMC article.
References
-
- Nevala-Plagemann, C., Hidalgo, M. & Garrido-Laguna, I. From state-of-the-art treatments to novel therapies for advanced-stage pancreatic cancer. Nat. Rev. Clin. Oncol.17, 108–123 (2020). - PubMed
-
- Conroy, T. et al. FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N. Engl. J. Med.379, 2395–2406 (2018). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

