Essential elements of radical pair magnetosensitivity in Drosophila
- PMID: 36813962
- PMCID: PMC9977682
- DOI: 10.1038/s41586-023-05735-z
Essential elements of radical pair magnetosensitivity in Drosophila
Erratum in
-
Publisher Correction: Essential elements of radical pair magnetosensitivity in Drosophila.Nature. 2023 Mar;615(7954):E27. doi: 10.1038/s41586-023-05929-5. Nature. 2023. PMID: 36922600 Free PMC article. No abstract available.
Abstract
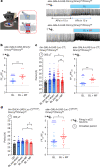
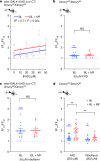
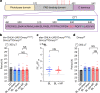
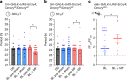
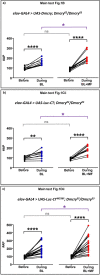
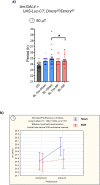
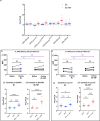
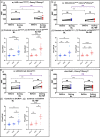
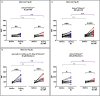
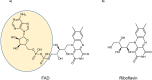
Many animals use Earth's magnetic field (also known as the geomagnetic field) for navigation1. The favoured mechanism for magnetosensitivity involves a blue-light-activated electron-transfer reaction between flavin adenine dinucleotide (FAD) and a chain of tryptophan residues within the photoreceptor protein CRYPTOCHROME (CRY). The spin-state of the resultant radical pair, and therefore the concentration of CRY in its active state, is influenced by the geomagnetic field2. However, the canonical CRY-centric radical-pair mechanism does not explain many physiological and behavioural observations2-8. Here, using electrophysiology and behavioural analyses, we assay magnetic-field responses at the single-neuron and organismal levels. We show that the 52 C-terminal amino acid residues of Drosophila melanogaster CRY, lacking the canonical FAD-binding domain and tryptophan chain, are sufficient to facilitate magnetoreception. We also show that increasing intracellular FAD potentiates both blue-light-induced and magnetic-field-dependent effects on the activity mediated by the C terminus. High levels of FAD alone are sufficient to cause blue-light neuronal sensitivity and, notably, the potentiation of this response in the co-presence of a magnetic field. These results reveal the essential components of a primary magnetoreceptor in flies, providing strong evidence that non-canonical (that is, non-CRY-dependent) radical pairs can elicit magnetic-field responses in cells.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

