Individuals with a previous symptomatic COVID-19 infection have altered heart rate and blood pressure variability during acute exercise
- PMID: 36814473
- PMCID: PMC9939691
- DOI: 10.3389/fphys.2023.1052369
Individuals with a previous symptomatic COVID-19 infection have altered heart rate and blood pressure variability during acute exercise
Abstract
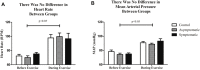
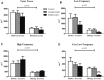
Introduction: As the number of COVID-19 cases begin to diminish it is important to turn our attention to any long-term issues that may be associated with a prior infection. Cardiovascular defects have been noted following prior SARS-CoV-2 infections. However, less is known about how a previous infection alters the cardiovascular response to exercise. Further, differences may exist during exercise between previously SARS-CoV-2 positive individuals who had symptoms (symptomatic) relative to those who did not have symptoms (asymptomatic). We hypothesized that previously symptomatic (S) COVID-19 recoveries have an altered cardiovascular response to acute exercise relative to both control (CON; never infected), and previously COVID-19 positive asymptomatic (AS) individuals. Methods: Twenty-seven subjects (CON = 9; AS = 9; S = 9) underwent 30 min of submaximal treadmill exercise. During exercise, blood pressure was recorded on the brachial artery every 5 min and 3-lead electrocardiography was measured continuously. Indirect indicators of autonomic nervous system health: heart rate variability and blood pressure variability were measured during each session. Baseline mean arterial pressure (MAP) was taken prior to exercise in seated, standing and supine positions. Results: Blood pressure was similar (p > 0.05) amongst all three groups. There were no differences between average heart rate (HR; CON = 104 ± 4 BPM vs AS = 118 ± 6 BPM vs. S = 112 ± 3 BPM), mean arterial pressure (MAP; CON = 108 ± 4 mmHg vs. AS = 105 ± 13 mmHg vs. S = 108 ± 7 mmHg) or oxygen consumption (VO2) between groups during a bout of exercise. However, the standard deviation of the inter beat intervals of normal sinus beats, a measure of heart rate variability (HRV) (CON = 138 ± 2.8 m vs. AS = 156 ± 6 m vs. S = 77.7 ± 11 m; p < 0.05) and blood pressure variability (BPV; CON = 5.18 ± 1.1 vs. AS = 12.1 ± 0.88 mmHg vs. S = 10.2 ± 10.7 mmHg; p < 0.05) were different in our S group. Further, when HRV was assessed in the frequency domain the very low frequency was different during exercise in the S group relative to the other groups. Discussion: Collectively, these data suggest that a previous symptomatic SARS-CoV-2 infection may alter heart rate and blood pressure regulation during exercise.
Keywords: COVID-19; autonomic nervous system; blood pressure variability; cardiovascular; exercise; heart rate variability.
Copyright © 2023 Chan, Senior, Homitz, Cashin and Guers.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Carney R. M., Freedland K. E., Stein P. K., Miller G. E., Steinmeyer B., Rich M. W., et al. (2007). Heart rate variability and markers of inflammation and coagulation in depressed patients with coronary heart disease. J. Psychosom. Res. 62, 463–467. 10.1016/j.jpsychores.2006.12.004 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

