The use of remimazolam versus propofol for induction and maintenance of general anesthesia: A systematic review and meta-analysis
- PMID: 36814492
- PMCID: PMC9939642
- DOI: 10.3389/fphar.2023.1101728
The use of remimazolam versus propofol for induction and maintenance of general anesthesia: A systematic review and meta-analysis
Abstract
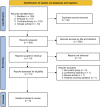
Background: The primary objective of this study was to compare the risk of hypotension, as well as the induction and recovery characteristics between remimazolam and propofol in patients receiving surgery under general anesthesia. Methods: The Embase, Medline, Google scholar, and the Cochrane Library databases were searched from inception to March 2022 for randomized controlled trials The primary outcome was the risk of post-induction hypotension between the two agents, while the secondary outcomes included anesthetic depth, induction efficacy, time to loss of consciousness (LOC), hemodynamic profiles, time to eye opening, extubation time as well as the incidence of injection pain and postoperative nausea/vomiting (PONV). Results: Meta-analysis of eight studies published from 2020 to 2022 involving 738 patients revealed a significantly lower risk of post-induction hypotension with the use of remimazolam compared to that with propofol [risk ratio (RR) = 0.57, 95% confidence interval (CI): 0.43 to 0.75, p < 0.0001, I2 = 12%, five studies, 564 patients]. After anesthetic induction, the anesthetic depth measured by bispectral index (BIS) was lighter in the remimazolam group than that in the propofol group (MD = 9.26, 95% confidence interval: 3.06 to 15.47, p = 0.003, I2 = 94%, five studies, 490 patients). The time to loss of consciousness was also longer in the former compared to the latter (MD = 15.49 s, 95%CI: 6.53 to 24.46, p = 0.0007, I2 = 61%, three studies, 331 patients). However, the use of remimazolam correlated with a lower risk of injection pain (RR = 0.03, 95%CI: 0.01 to 0.16, p < 0.0001, I2 = 0%, three studies, 407 patients) despite comparable efficacy of anesthetic induction (RR = 0.98, 95%CI: 0.9 to 1.06, p = 0.57, I2 = 76%, two studies, 319 patients). Our results demonstrated no difference in time to eye opening, extubation time, and risk of PONV between the two groups. Conclusion: Remimazolam was associated with a lower risk of post-induction hypotension after anesthetic induction compared with propofol with similar recovery characteristics. Further studies are required to support our findings. Systematic Review Registration: https://www.crd.york.ac.uk/prospero/; Identifier: CRD42022320658.
Keywords: anesthesia; general anesthesia (GA); hypotension; propofol; remimazolam.
Copyright © 2023 Ko, Hung, Illias, Chiu, Yu, Lin, Chen and Sun.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Ahuja S., Mascha E. J., Yang D., Maheshwari K., Cohen B., Khanna A. K., et al. (2020). Associations of intraoperative radial arterial systolic, diastolic, mean, and pulse pressures with myocardial and acute kidney injury after noncardiac surgery: A retrospective cohort analysis. Anesthesiology 132, 291–306. 10.1097/ALN.0000000000003048 - DOI - PubMed
-
- Antonik L. J., Goldwater D. R., Kilpatrick G. J., Tilbrook G. S., Borkett K. M. (2012). A placebo- and midazolam-controlled phase I single ascending-dose study evaluating the safety, pharmacokinetics, and pharmacodynamics of remimazolam (CNS 7056): Part I. Safety, efficacy, and basic pharmacokinetics. Anesth. analgesia 115, 274–283. 10.1213/ANE.0b013e31823f0c28 - DOI - PubMed
-
- Belcher A. W., Leung S., Cohen B., Yang D., Mascha E. J., Turan A., et al. (2017). Incidence of complications in the post-anesthesia care unit and associated healthcare utilization in patients undergoing non-cardiac surgery requiring neuromuscular blockade 2005–2013: A single center study. J. Clin. Anesth. 43, 33–38. 10.1016/j.jclinane.2017.09.005 - DOI - PubMed
-
- Borkett K. M., Riff D. S., Schwartz H. I., Winkle P. J., Pambianco D. J., Lees J. P., et al. (2015). A Phase IIa, randomized, double-blind study of remimazolam (CNS 7056) versus midazolam for sedation in upper gastrointestinal endoscopy. Anesth. analgesia 120, 771–780. 10.1213/ANE.0000000000000548 - DOI - PubMed
-
- Çaparlar C., Özhan M., Süzer M. A., Yazicioğlu D., Eşkin M. B., Şenkal S., et al. (2017). Fast-track anesthesia in patients undergoing outpatient laparoscopic cholecystectomy: Comparison of sevoflurane with total intravenous anesthesia. J. Clin. Anesth. 37, 25–30. 10.1016/j.jclinane.2016.10.036 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

